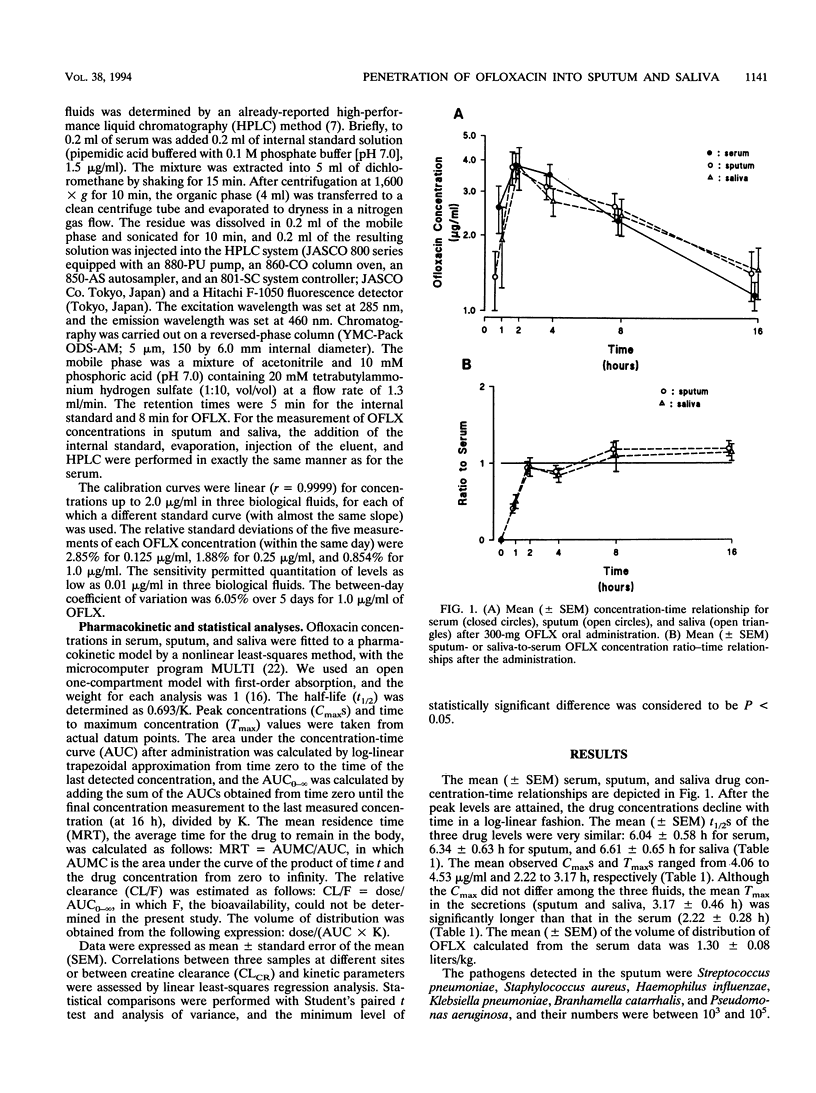
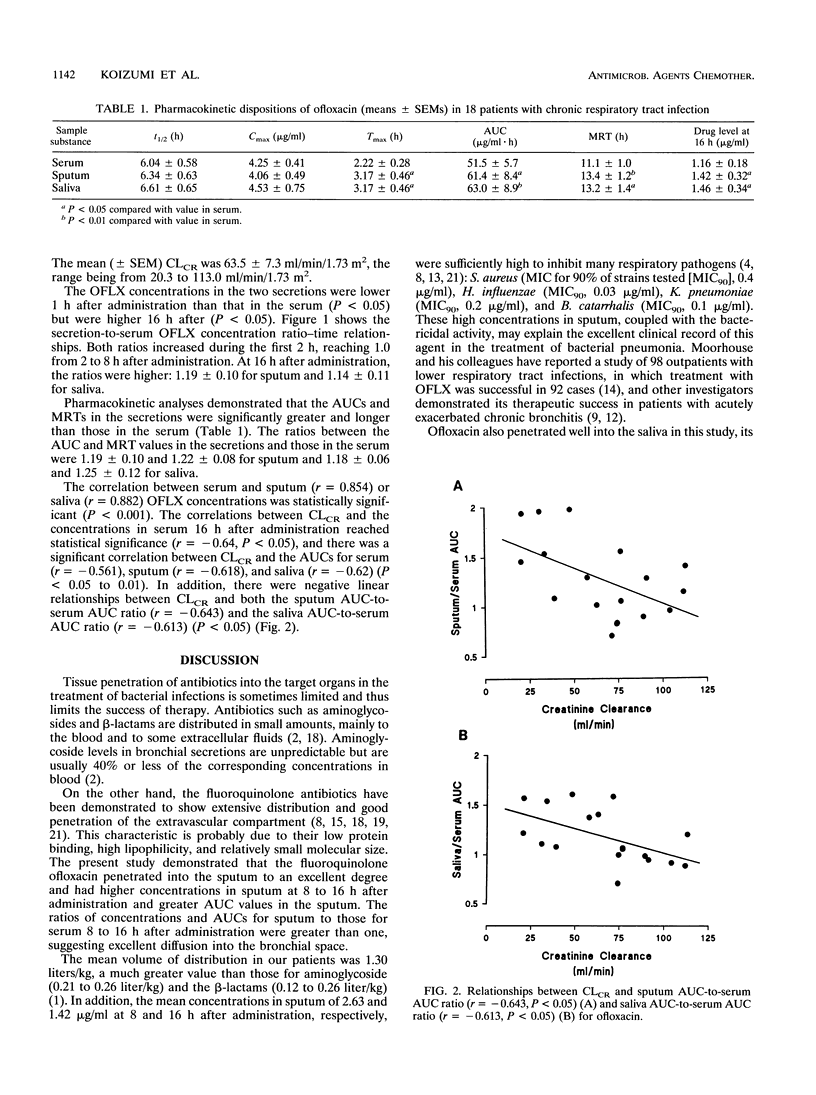
Abstract
To ascertain whether monitoring of the concentrations of ofloxacin in saliva during a course of treatment is more suitable and safer than that of its levels in blood, we simultaneously monitored its concentrations in three body fluids (blood, saliva, and expectorated sputum) after a 300-mg administration in 18 patients with chronic respiratory infection. The mean (+/- standard error of the mean) half-lives derived from the three drug level-time relationships were similar: 6.04 +/- 0.58 h for serum, 6.34 +/- 0.63 h for sputum, and 6.61 +/- 0.65 h for saliva. The mean peak concentration (4.06 to 4.53 micrograms/ml) did not differ at the three sites, but the times taken to reach peak concentration in saliva and sputum (3.17 +/- 0.46 h) were significantly longer than that in serum (2.22 +/- 0.28 h). The ratios of the concentrations in saliva and sputum to the concentration in serum increased during the first 2 h and reached 1.0 between 2 and 8 h after administration. They rose above 1.0 16 h after administration: 1.14 +/- 0.11 for saliva and 1.19 +/- 0.10 for sputum. The concentration-time relationship for sputum corresponded closely with the concentration-time relationship for saliva, and an overall significant correlation between the concentrations in sputum and saliva was obtained (P < 0.01). These results suggest that monitoring concentrations in saliva may be more valid, as well as less invasive, than monitoring of the levels in blood for ensuring that the drug concentration reaches its therapeutic level in bronchial secretions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Edson R. S., Keys T. F. The aminoglycosides. Streptomycin, kanamycin, gentamicin, tobramycin, amikacin, netilmicin, sisomicin. Mayo Clin Proc. 1983 Feb;58(2):99–102. [PubMed] [Google Scholar]
- Eliopoulos G. M. New quinolones: pharmacology, pharmacokinetics, and dosing in patients with renal insufficiency. Rev Infect Dis. 1988 Jan-Feb;10 (Suppl 1):S102–S105. doi: 10.1093/clinids/10.supplement_1.s102. [DOI] [PubMed] [Google Scholar]
- Fillastre J. P., Leroy A., Humbert G. Ofloxacin pharmacokinetics in renal failure. Antimicrob Agents Chemother. 1987 Feb;31(2):156–160. doi: 10.1128/aac.31.2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G. G. A multicenter study on clinical efficacy of ofloxacin in respiratory and urinary tract infections. Infection. 1986;14 (Suppl 4):S300–S302. doi: 10.1007/BF01661299. [DOI] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. Fluoroquinolone antimicrobial agents. N Engl J Med. 1991 Feb 7;324(6):384–394. doi: 10.1056/NEJM199102073240606. [DOI] [PubMed] [Google Scholar]
- Lockley M. R., Wise R., Dent J. The pharmacokinetics and tissue penetration of ofloxacin. J Antimicrob Chemother. 1984 Dec;14(6):647–652. doi: 10.1093/jac/14.6.647. [DOI] [PubMed] [Google Scholar]
- Lode H., Höffken G., Olschewski P., Sievers B., Kirch A., Borner K., Koeppe P. Pharmacokinetics of ofloxacin after parenteral and oral administration. Antimicrob Agents Chemother. 1987 Sep;31(9):1338–1342. doi: 10.1128/aac.31.9.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maesen F. P., Davies B. I., Teengs J. P., Baur C. Quinolone antimicrobial agents in acute exacerbations of chronic bronchitis. Eur J Respir Dis Suppl. 1986;146:585–590. [PubMed] [Google Scholar]
- Monk J. P., Campoli-Richards D. M. Ofloxacin. A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs. 1987 Apr;33(4):346–391. doi: 10.2165/00003495-198733040-00003. [DOI] [PubMed] [Google Scholar]
- Moorhouse E. C., Clarke P. C. Efficacy of ofloxacin in the treatment of lower respiratory tract infections in general practice. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):135–138. doi: 10.1093/jac/22.supplement_c.135. [DOI] [PubMed] [Google Scholar]
- Nikaido H., Thanassi D. G. Penetration of lipophilic agents with multiple protonation sites into bacterial cells: tetracyclines and fluoroquinolones as examples. Antimicrob Agents Chemother. 1993 Jul;37(7):1393–1399. doi: 10.1128/aac.37.7.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi A., Ko Y., Fujihara H., Miyamoto G., Okada K., Odomi M. Pharmacokinetics, safety, and pharmacologic effects of OPC-21268, a nonpeptide orally active vasopressin V1 receptor antagonist, in humans. J Clin Pharmacol. 1993 Mar;33(3):230–238. doi: 10.1002/j.1552-4604.1993.tb03949.x. [DOI] [PubMed] [Google Scholar]
- Schentag J. J. Clinical significance of antibiotic tissue penetration. Clin Pharmacokinet. 1989;16 (Suppl 1):25–31. doi: 10.2165/00003088-198900161-00005. [DOI] [PubMed] [Google Scholar]
- Sörgel F., Jaehde U., Naber K., Stephan U. Pharmacokinetic disposition of quinolones in human body fluids and tissues. Clin Pharmacokinet. 1989;16 (Suppl 1):5–24. doi: 10.2165/00003088-198900161-00004. [DOI] [PubMed] [Google Scholar]
- Wise R., Lockley M. R. The pharmacokinetics of ofloxacin and a review of its tissue penetration. J Antimicrob Chemother. 1988 Sep;22 (Suppl 100):59–64. doi: 10.1093/jac/22.supplement_c.59. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Fluoroquinolone antimicrobial agents. Clin Microbiol Rev. 1989 Oct;2(4):378–424. doi: 10.1128/cmr.2.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka K., Tanigawara Y., Nakagawa T., Uno T. A pharmacokinetic analysis program (multi) for microcomputer. J Pharmacobiodyn. 1981 Nov;4(11):879–885. doi: 10.1248/bpb1978.4.879. [DOI] [PubMed] [Google Scholar]



