Abstract
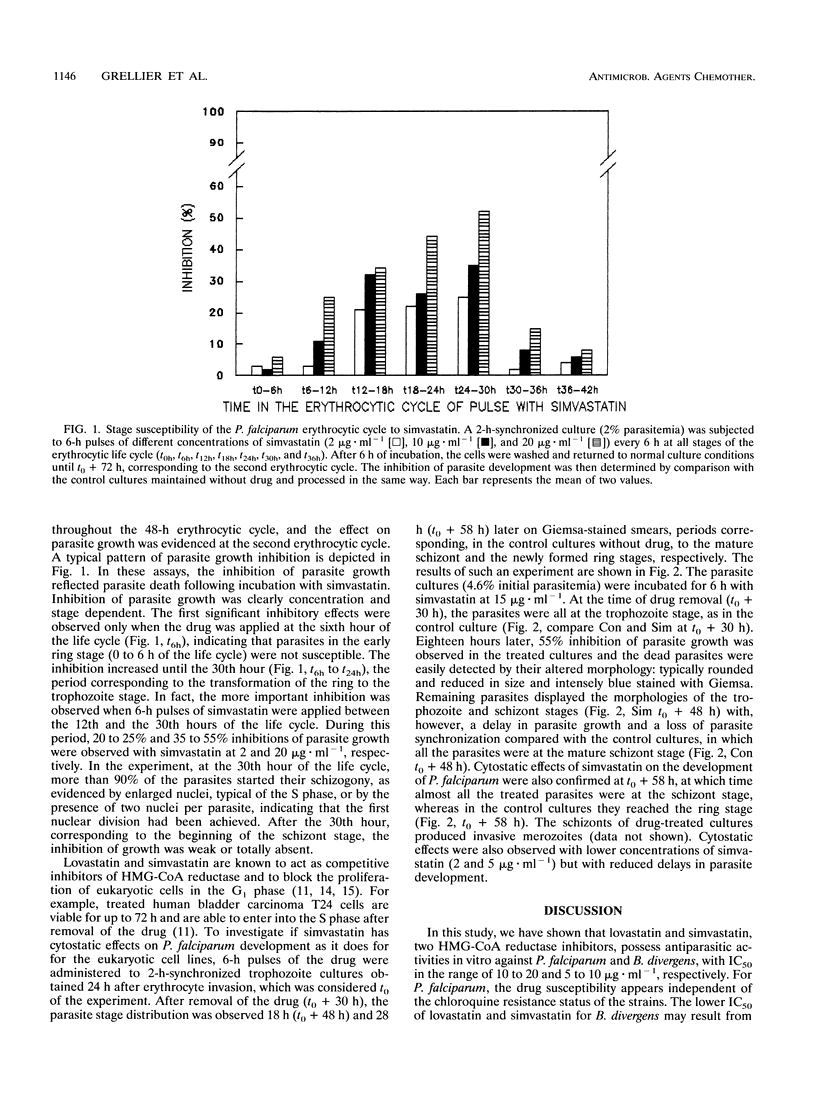
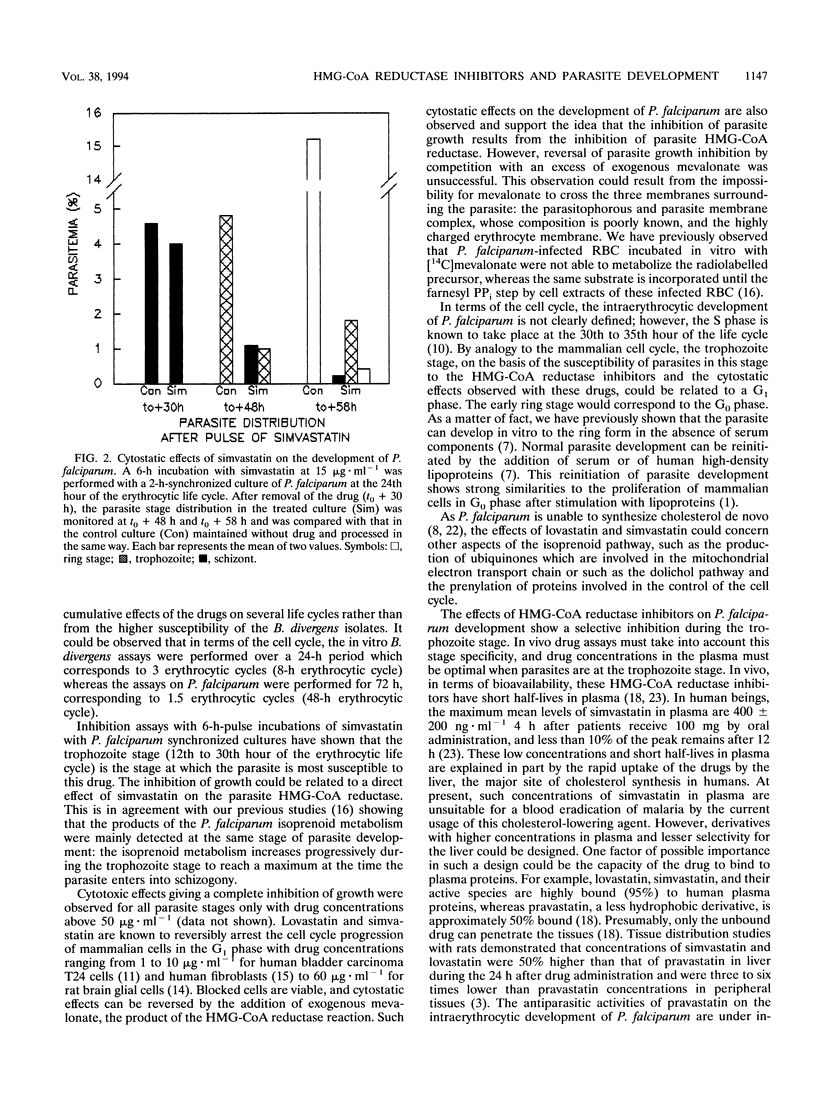
The 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors lovastatin and simvastatin inhibit the in vitro intraerythrocytic development of Plasmodium falciparum and Babesia divergens, with concentrations inhibiting parasite growth by 50% in the ranges of 10 to 20 and 5 to 10 micrograms.ml-1, respectively. For P. falciparum, the 50% inhibitory concentrations were in the same range whatever the chloroquine susceptibility of the strains tested (strain F32/Tanzania [chloroquine susceptible] or FcB.1/Columbia [resistant]). The stage-dependent susceptibility of P. falciparum to simvastatin was studied by subjecting synchronized cultures to 6-h pulses of drug throughout the 48-h erythrocytic life cycle. The most important inhibitory effects were observed between the 12th and 30th hours of the cycle, corresponding to the trophozoite stage. This period precedes the S phase and the nuclear divisions. Parasites in the newly formed ring stage (time zero to the 6th hour of the cycle) and the schizont stage (30th to 48th hour of the cycle) were weakly or not susceptible to simvastatin pulses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuthbert J. A., Lipsky P. E. Lipoproteins may provide fatty acids necessary for human lymphocyte proliferation by both low density lipoprotein receptor-dependent and -independent mechanisms. J Biol Chem. 1989 Aug 15;264(23):13468–13474. [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Regulation of the mevalonate pathway. Nature. 1990 Feb 1;343(6257):425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- Gorenflot A., Brasseur P., Precigout E., L'Hostis M., Marchand A., Schrevel J. Cytological and immunological responses to Babesia divergens in different hosts: ox, gerbil, man. Parasitol Res. 1991;77(1):3–12. doi: 10.1007/BF00934377. [DOI] [PubMed] [Google Scholar]
- Grellier P., Rigomier D., Clavey V., Fruchart J. C., Schrevel J. Lipid traffic between high density lipoproteins and Plasmodium falciparum-infected red blood cells. J Cell Biol. 1991 Jan;112(2):267–277. doi: 10.1083/jcb.112.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grellier P., Rigomier D., Schrével J. Induction in vitro de la schizogonie de Plasmodium falciparum par les lipoprotéines humaines de haute densité (HDL). C R Acad Sci III. 1990;311(10):361–367. [PubMed] [Google Scholar]
- Holz G. G., Jr Lipids and the malarial parasite. Bull World Health Organ. 1977;55(2-3):237–248. [PMC free article] [PubMed] [Google Scholar]
- Howard R. J., Battye F. L., Mitchell G. F. Plasmodium-infected blood cells analyzed and sorted by flow fluorimetry with the deoxyribonucleic acid binding dye 33258 Hoechst. J Histochem Cytochem. 1979 Apr;27(4):803–813. doi: 10.1177/27.4.87413. [DOI] [PubMed] [Google Scholar]
- Inselburg J., Banyal H. S. Synthesis of DNA during the asexual cycle of Plasmodium falciparum in culture. Mol Biochem Parasitol. 1984 Jan;10(1):79–87. doi: 10.1016/0166-6851(84)90020-3. [DOI] [PubMed] [Google Scholar]
- Jakóbisiak M., Bruno S., Skierski J. S., Darzynkiewicz Z. Cell cycle-specific effects of lovastatin. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3628–3632. doi: 10.1073/pnas.88.9.3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Langan T. J., Volpe J. J. Cell cycle-specific requirement for mevalonate, but not for cholesterol, for DNA synthesis in glial primary cultures. J Neurochem. 1987 Aug;49(2):513–521. doi: 10.1111/j.1471-4159.1987.tb02894.x. [DOI] [PubMed] [Google Scholar]
- Larsson O., Wejde J. Dolichol delays G1-arrest for one cell cycle in human fibroblasts subjected to depletion of serum or mevalonate. J Cell Sci. 1992 Dec;103(Pt 4):1065–1072. doi: 10.1242/jcs.103.4.1065. [DOI] [PubMed] [Google Scholar]
- Mbaya B., Rigomier D., Edorh G. G., Karst F., Schrevel J. Isoprenoid metabolism in Plasmodium falciparum during the intraerythrocytic phase of malaria. Biochem Biophys Res Commun. 1990 Dec 31;173(3):849–854. doi: 10.1016/s0006-291x(05)80864-2. [DOI] [PubMed] [Google Scholar]
- Pasvol G., Wilson R. J., Smalley M. E., Brown J. Separation of viable schizont-infected red cells of Plasmodium falciparum from human blood. Ann Trop Med Parasitol. 1978 Feb;72(1):87–88. doi: 10.1080/00034983.1978.11719283. [DOI] [PubMed] [Google Scholar]
- Pentikainen P. J., Saraheimo M., Schwartz J. I., Amin R. D., Schwartz M. S., Brunner-Ferber F., Rogers J. D. Comparative pharmacokinetics of lovastatin, simvastatin and pravastatin in humans. J Clin Pharmacol. 1992 Feb;32(2):136–140. doi: 10.1002/j.1552-4604.1992.tb03818.x. [DOI] [PubMed] [Google Scholar]
- Precigout E., Gorenflot A., Valentin A., Bissuel G., Carcy B., Brasseur P., Moreau Y., Schrevel J. Analysis of immune responses of different hosts to Babesia divergens isolates from different geographic areas and capacity of culture-derived exoantigens to induce efficient cross-protection. Infect Immun. 1991 Aug;59(8):2799–2805. doi: 10.1128/iai.59.8.2799-2805.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Valentin A., Rigomier D., Précigout E., Carcy B., Gorenflot A., Schrével J. Lipid trafficking between high density lipoproteins and Babesia divergens-infected human erythrocytes. Biol Cell. 1991;73(1):63–70. doi: 10.1016/0248-4900(91)90010-k. [DOI] [PubMed] [Google Scholar]
- Vial H. J., Philippot J. R., Wallach D. F. A reevaluation of the status of cholesterol in erythrocytes infected by Plasmodium knowlesi and P. falciparum. Mol Biochem Parasitol. 1984 Sep;13(1):53–65. doi: 10.1016/0166-6851(84)90101-4. [DOI] [PubMed] [Google Scholar]
- Vickers S., Duncan C. A., Chen I. W., Rosegay A., Duggan D. E. Metabolic disposition studies on simvastatin, a cholesterol-lowering prodrug. Drug Metab Dispos. 1990 Mar-Apr;18(2):138–145. [PubMed] [Google Scholar]



