Abstract
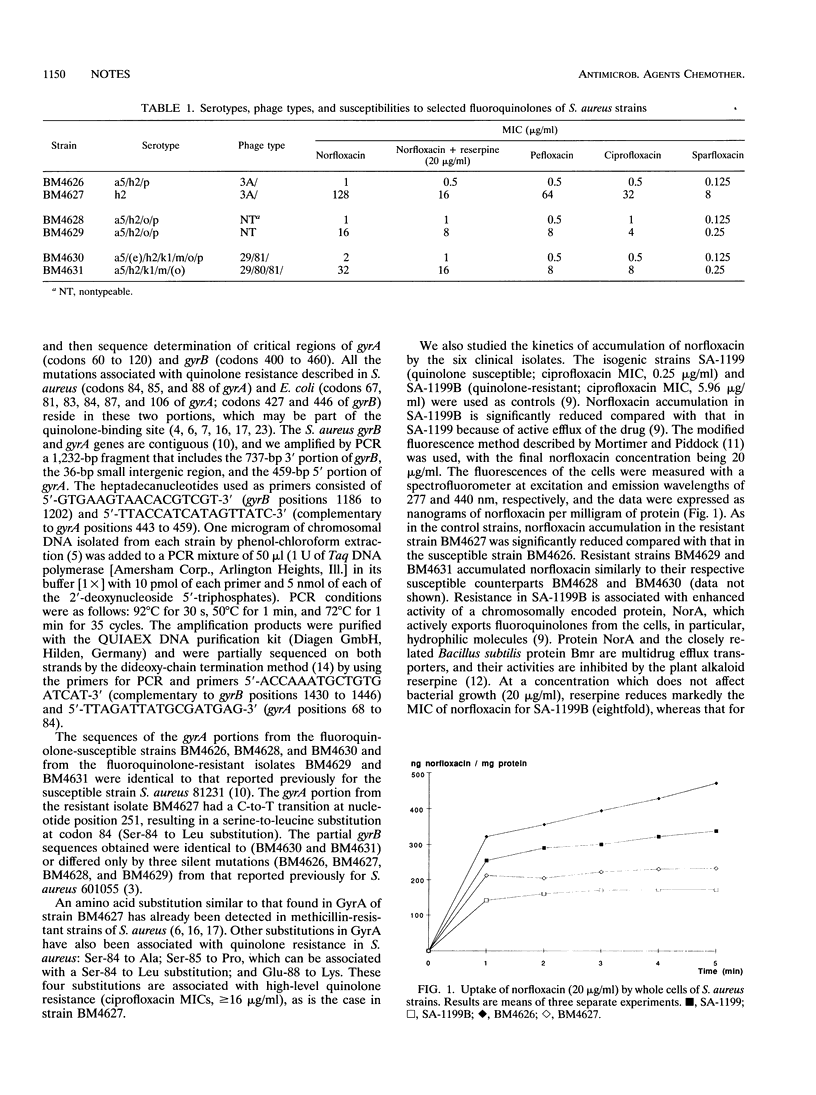
Staphylococcus aureus BM4626 (ciprofloxacin MIC, 0.5 microgram/ml) and BM4627 (ciprofloxacin MIC, 32 microgram/ml) were isolated from the same patient before and during pefloxcin therapy for septic tibial nonunion, respectively. The two strains had similar serotypes and indistinguishable phage types and SmaI-generated restriction fragment length polymorphisms. Portions of the gyrA (codons 60 to 120) and the gyrB (codons 420 to 480) genes of each clinical isolate were amplified by PCR and sequenced. Strain BM4627 had a serine-to-leucine substitution resulting from a cytosine-to-thymidine mutation at codon 84 of gyrA relative to the sequence of the gyrA gene of BM4626. Norfloxacin accumulation, measured in a whole-cell uptake assay, was significantly lower in BM4627 than BM4626. These data indicate that double mutants can be selected in vivo under fluoroquinolone therapy.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Pfaller M. A., Fuchs P. C. Spontaneously occurring staphylococcal mutants resistant to clinically achievable concentrations of ciprofloxacin and temafloxacin. Eur J Clin Microbiol Infect Dis. 1992 Mar;11(3):243–246. doi: 10.1007/BF02098088. [DOI] [PubMed] [Google Scholar]
- Brockbank S. M., Barth P. T. Cloning, sequencing, and expression of the DNA gyrase genes from Staphylococcus aureus. J Bacteriol. 1993 Jun;175(11):3269–3277. doi: 10.1128/jb.175.11.3269-3277.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambau E., Bordon F., Collatz E., Gutmann L. Novel gyrA point mutation in a strain of Escherichia coli resistant to fluoroquinolones but not to nalidixic acid. Antimicrob Agents Chemother. 1993 Jun;37(6):1247–1252. doi: 10.1128/aac.37.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesneau O., Morvan A., Grimont F., Labischinski H., el Solh N. Staphylococcus pasteuri sp. nov., isolated from human, animal, and food specimens. Int J Syst Bacteriol. 1993 Apr;43(2):237–244. doi: 10.1099/00207713-43-2-237. [DOI] [PubMed] [Google Scholar]
- Goswitz J. J., Willard K. E., Fasching C. E., Peterson L. R. Detection of gyrA gene mutations associated with ciprofloxacin resistance in methicillin-resistant Staphylococcus aureus: analysis by polymerase chain reaction and automated direct DNA sequencing. Antimicrob Agents Chemother. 1992 May;36(5):1166–1169. doi: 10.1128/aac.36.5.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P., Maxwell A. Novel quinolone resistance mutations of the Escherichia coli DNA gyrase A protein: enzymatic analysis of the mutant proteins. Antimicrob Agents Chemother. 1991 Feb;35(2):335–340. doi: 10.1128/aac.35.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori S., Ohshita Y., Utsui Y., Hiramatsu K. Sequential acquisition of norfloxacin and ofloxacin resistance by methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1993 Nov;37(11):2278–2284. doi: 10.1128/aac.37.11.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Ruble C. A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993 May;37(5):1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margerrison E. E., Hopewell R., Fisher L. M. Nucleotide sequence of the Staphylococcus aureus gyrB-gyrA locus encoding the DNA gyrase A and B proteins. J Bacteriol. 1992 Mar;174(5):1596–1603. doi: 10.1128/jb.174.5.1596-1603.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. G., Piddock L. J. A comparison of methods used for measuring the accumulation of quinolones by Enterobacteriaceae, Pseudomonas aeruginosa and Staphylococcus aureus. J Antimicrob Chemother. 1991 Nov;28(5):639–653. doi: 10.1093/jac/28.5.639. [DOI] [PubMed] [Google Scholar]
- Neyfakh A. A., Borsch C. M., Kaatz G. W. Fluoroquinolone resistance protein NorA of Staphylococcus aureus is a multidrug efflux transporter. Antimicrob Agents Chemother. 1993 Jan;37(1):128–129. doi: 10.1128/aac.37.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OEDING P. Serological typing of Staphylococci. Acta Pathol Microbiol Scand Suppl. 1952 Jun;93:356–365. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalit I., Berger S. A., Gorea A., Frimerman H. Widespread quinolone resistance among methicillin-resistant Staphylococcus aureus isolates in a general hospital. Antimicrob Agents Chemother. 1989 Apr;33(4):593–594. doi: 10.1128/aac.33.4.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S., Oram M., Jensen B., Peterson L. R., Fisher L. M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S., Peterson L. R., Fisher L. M. Ciprofloxacin resistance in coagulase-positive and -negative staphylococci: role of mutations at serine 84 in the DNA gyrase A protein of Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother. 1991 Oct;35(10):2151–2154. doi: 10.1128/aac.35.10.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struelens M. J., Deplano A., Godard C., Maes N., Serruys E. Epidemiologic typing and delineation of genetic relatedness of methicillin-resistant Staphylococcus aureus by macrorestriction analysis of genomic DNA by using pulsed-field gel electrophoresis. J Clin Microbiol. 1992 Oct;30(10):2599–2605. doi: 10.1128/jcm.30.10.2599-2605.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trucksis M., Wolfson J. S., Hooper D. C. A novel locus conferring fluoroquinolone resistance in Staphylococcus aureus. J Bacteriol. 1991 Sep;173(18):5854–5860. doi: 10.1128/jb.173.18.5854-5860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura S., Ubukata K., Konno M. Nucleotide sequence and characterization of the Staphylococcus aureus norA gene, which confers resistance to quinolones. J Bacteriol. 1990 Dec;172(12):6942–6949. doi: 10.1128/jb.172.12.6942-6949.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]


