Abstract
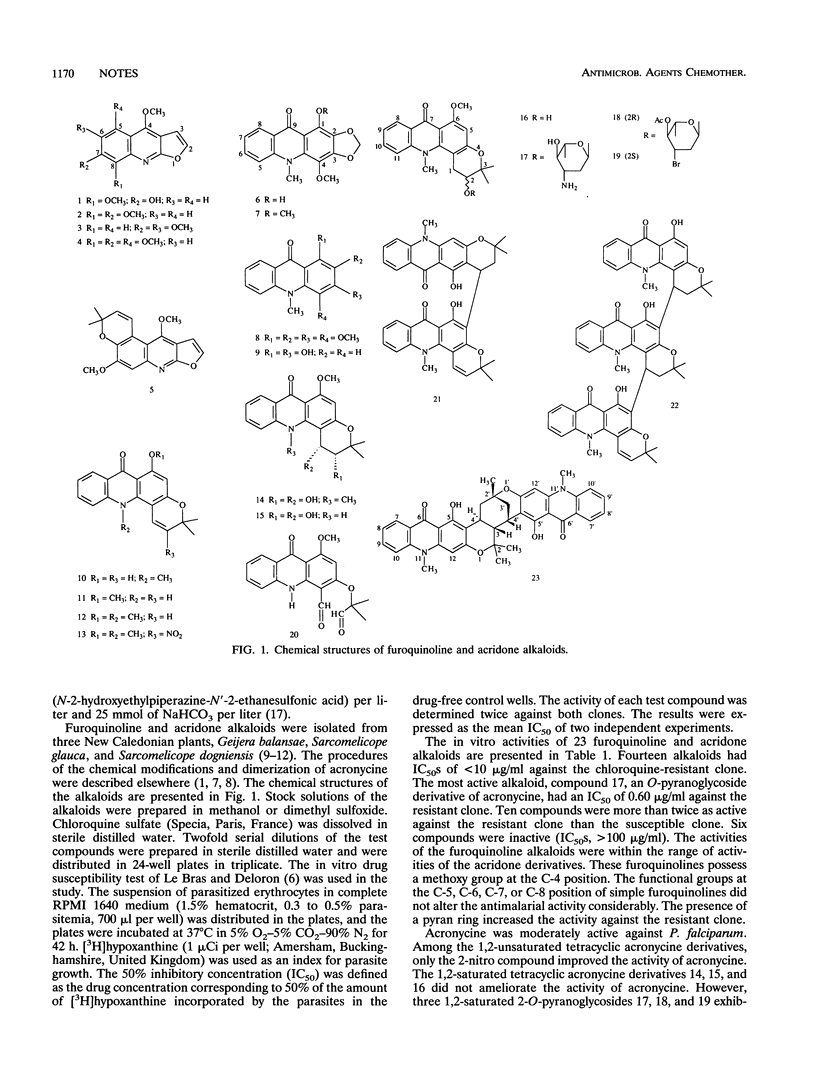
The in vitro activities of furo[2,3b]quinoline and acridone alkaloids against Plasmodium falciparum were evaluated by an isotopic semimicrotest. A pyran ring in the furoquinoline nucleus and 2-O-pyranoglycoside and 2-nitro substituents in the acridone nucleus improved the antimalarial activities of the compounds. These findings provide a clue for further chemical modifications.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cruickshank P. A., Lee F. T., Lupichuk A. Antimalarials. 1. Aminoalkylamino derivatives of 2,3-dihydrofuroquinolines. J Med Chem. 1970 Nov;13(6):1110–1114. doi: 10.1021/jm00300a022. [DOI] [PubMed] [Google Scholar]
- Fujioka H., Kato N., Fujita M., Fujimura K., Nishiyama Y. Activities of new acridone alkaloid derivatives against Plasmodium yoelii in vitro. Arzneimittelforschung. 1990 Sep;40(9):1026–1029. [PubMed] [Google Scholar]
- Fujioka H., Nishiyama Y., Furukawa H., Kumada N. In vitro and in vivo activities of atalaphillinine and related acridone alkaloids against rodent malaria. Antimicrob Agents Chemother. 1989 Jan;33(1):6–9. doi: 10.1128/aac.33.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras J., Deloron P. In vitro study of drug sensitivity of Plasmodium falciparum: evaluation of a new semi-micro test. Am J Trop Med Hyg. 1983 May;32(3):447–451. doi: 10.4269/ajtmh.1983.32.447. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Skaltsounis A. L., Tillequin F., Koch M. Chemistry of Acronycine; Part 1. Synthesis of 1-Hydroxy-1,2-dihydroacronycine and 2-Hydroxy-1,2-dihydroacronycine. Planta Med. 1988 Feb;54(1):24–27. doi: 10.1055/s-2006-962323. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Skaltsounis A. L., Tillequin F., Koch M., Pusset J. Plantes de Nouvelle-Calédonie. Alcaloïdes des écorces de Sarcomelicope dogniensis Hartley. Ann Pharm Fr. 1989;47(3):149–156. [PubMed] [Google Scholar]
- Queener S. F., Fujioka H., Nishiyama Y., Furukawa H., Bartlett M. S., Smith J. W. In vitro activities of acridone alkaloids against Pneumocystis carinii. Antimicrob Agents Chemother. 1991 Feb;35(2):377–379. doi: 10.1128/aac.35.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarffe J. H., Beaumont A. R., Crowther D. Phase I-II evaluation of acronine in patients with multiple myeloma. Cancer Treat Rep. 1983 Jan;67(1):93–94. [PubMed] [Google Scholar]
- Schneider J., Evans E. L., Grunberg E., Fryer R. I. Synthesis and biological activity of acronycine analogs. J Med Chem. 1972 Mar;15(3):266–270. doi: 10.1021/jm00273a014. [DOI] [PubMed] [Google Scholar]
- Svoboda G. H., Poore G. A., Simpson P. J., Boder G. B. Alkaloids of Acronychia Baueri Schott I. Isolation of the alkaloids and a study of the antitumor and other biological properties of acronycine. J Pharm Sci. 1966 Aug;55(8):758–768. doi: 10.1002/jps.2600550803. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Wernsdorfer W. H., Payne D. The dynamics of drug resistance in Plasmodium falciparum. Pharmacol Ther. 1991;50(1):95–121. doi: 10.1016/0163-7258(91)90074-v. [DOI] [PubMed] [Google Scholar]


