Abstract
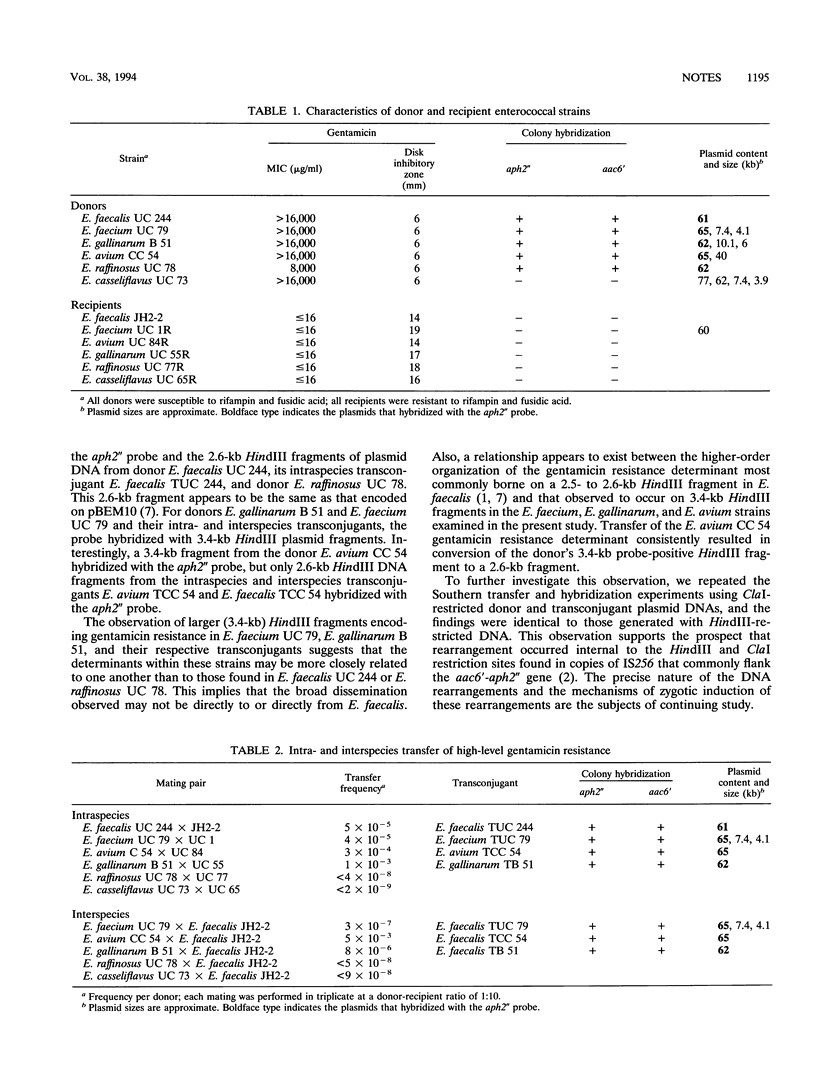
Gentamicin resistance in six enterococcal species was investigated. Transfer of resistance was observed for the donors E. faecium UC 79, E. avium CC 54, and E. gallinarum B 51, but not for E. raffinosus UC 78 or E. casseliflavus UC 73. Except for E. casseliflavus UC 73, homology was observed between the E. faecalis aac6'-aph2" gene and DNA from other species. Whereas 2.6-kb HindIII fragments encoded resistance in E. faecalis UC 244, its transconjugant, and E. raffinosus UC 78, 3.4-kb fragments encoded resistance in E. faecium UC 79, E. gallinarum B 51, and their transconjugants. A 3.4-kb fragment encoded resistance in E. avium CC 54, but 2.6-kb fragments encoded resistance in the transconjugants. Although many similarities were found among the strains, the heterogeneity in gentamicin resistance exhibited by some isolates indicates diversity among these determinants.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Byrne M. E., Gillespie M. T., Skurray R. A. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990 Nov;34(11):2106–2113. doi: 10.1128/aac.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M. E., Rouch D. A., Skurray R. A. Nucleotide sequence analysis of IS256 from the Staphylococcus aureus gentamicin-tobramycin-kanamycin-resistance transposon Tn4001. Gene. 1989 Sep 30;81(2):361–367. doi: 10.1016/0378-1119(89)90197-2. [DOI] [PubMed] [Google Scholar]
- Chen H. Y., Williams J. D. Transferable resistance and aminoglycoside-modifying enzymes in enterococci. J Med Microbiol. 1985 Oct;20(2):187–196. doi: 10.1099/00222615-20-2-187. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Tomich P. K., Gawron-Burke M. C., Franke A. E., Yagi Y., An F. Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982 Dec;152(3):1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C., Zighelboim-Daum S., Reiszner E., Goldmann D., Moellering R. C., Jr High-level resistance to gentamicin in clinical isolates of Streptococcus (Enterococcus) faecium. Antimicrob Agents Chemother. 1988 Oct;32(10):1528–1532. doi: 10.1128/aac.32.10.1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferretti J. J., Gilmore K. S., Courvalin P. Nucleotide sequence analysis of the gene specifying the bifunctional 6'-aminoglycoside acetyltransferase 2"-aminoglycoside phosphotransferase enzyme in Streptococcus faecalis and identification and cloning of gene regions specifying the two activities. J Bacteriol. 1986 Aug;167(2):631–638. doi: 10.1128/jb.167.2.631-638.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodel-Christian S. L., Murray B. E. Characterization of the gentamicin resistance transposon Tn5281 from Enterococcus faecalis and comparison to staphylococcal transposons Tn4001 and Tn4031. Antimicrob Agents Chemother. 1991 Jun;35(6):1147–1152. doi: 10.1128/aac.35.6.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A. E., Hobbs S. J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974 Feb;117(2):360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufhold A., Podbielski A., Horaud T., Ferrieri P. Identical genes confer high-level resistance to gentamicin upon Enterococcus faecalis, Enterococcus faecium, and Streptococcus agalactiae. Antimicrob Agents Chemother. 1992 Jun;36(6):1215–1218. doi: 10.1128/aac.36.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederski-Samoraj B. D., Murray B. E. High-level resistance to gentamicin in clinical isolates of enterococci. J Infect Dis. 1983 Apr;147(4):751–757. doi: 10.1093/infdis/147.4.751. [DOI] [PubMed] [Google Scholar]
- Ruoff K. L., de la Maza L., Murtagh M. J., Spargo J. D., Ferraro M. J. Species identities of enterococci isolated from clinical specimens. J Clin Microbiol. 1990 Mar;28(3):435–437. doi: 10.1128/jcm.28.3.435-437.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Boonlayangoor S., Schulz J. E. Detection of high-level aminoglycoside resistance in enterococci other than Enterococcus faecalis. J Clin Microbiol. 1991 Nov;29(11):2595–2598. doi: 10.1128/jcm.29.11.2595-2598.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. Effects of medium and inoculum variations on screening for high-level aminoglycoside resistance in Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):250–256. doi: 10.1128/jcm.26.2.250-256.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahm D. F., Torres C. High-content aminoglycoside disks for determining aminoglycoside-penicillin synergy against Enterococcus faecalis. J Clin Microbiol. 1988 Feb;26(2):257–260. doi: 10.1128/jcm.26.2.257-260.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weaver K. E., Clewell D. B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988 Sep;170(9):4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodford N., McNamara E., Smyth E., George R. C. High-level resistance to gentamicin in Enterococcus faecium. J Antimicrob Chemother. 1992 Apr;29(4):395–403. doi: 10.1093/jac/29.4.395. [DOI] [PubMed] [Google Scholar]



