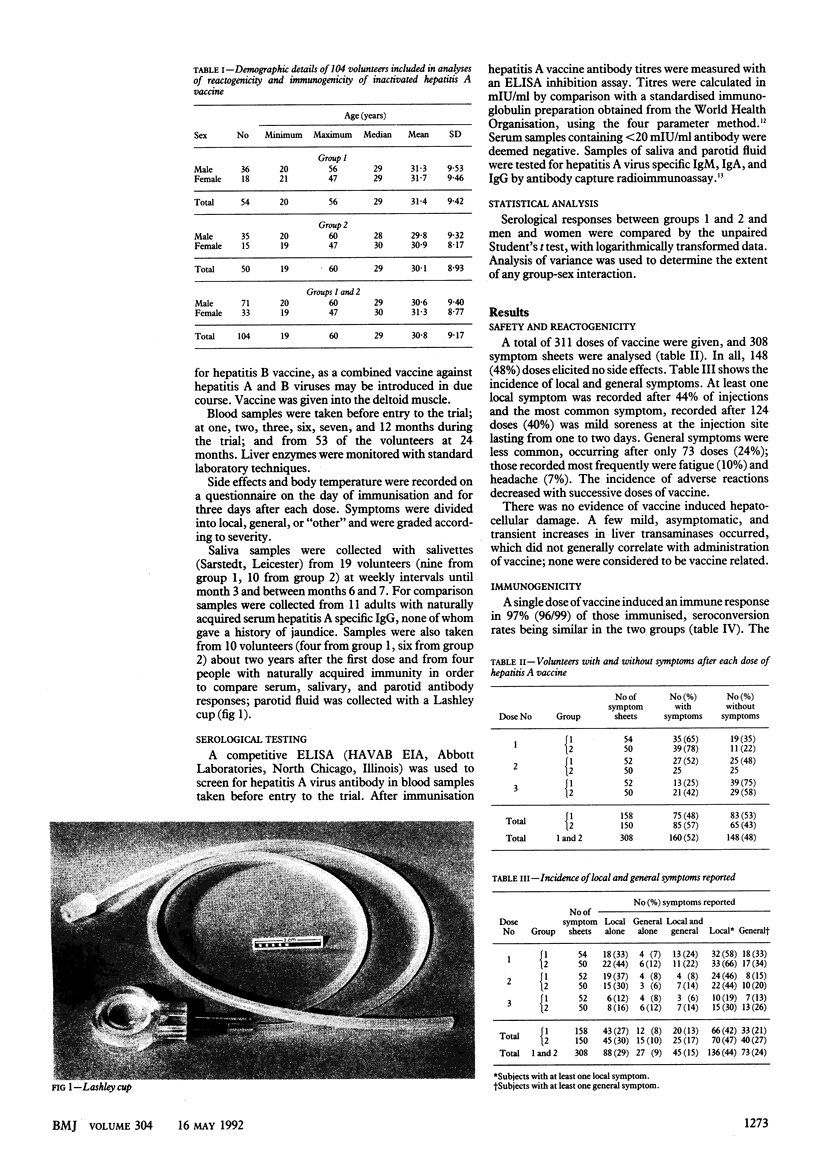
Abstract
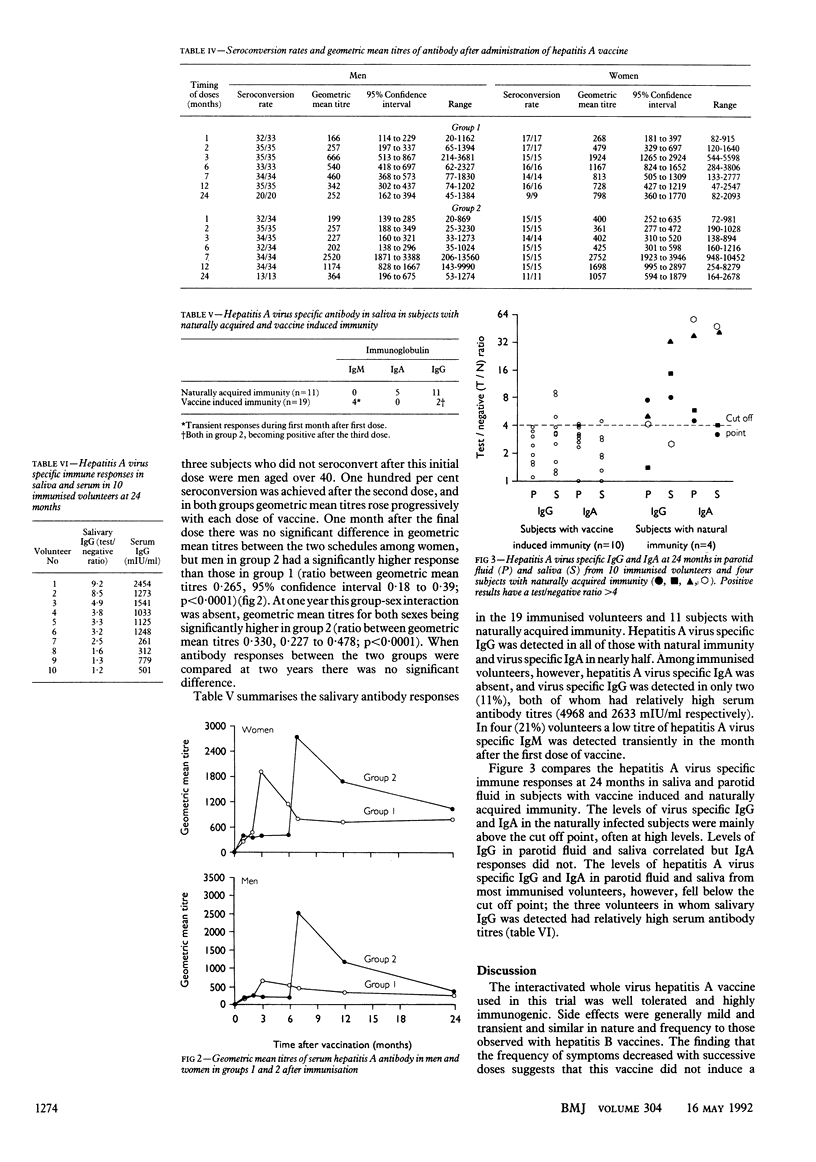
OBJECTIVE--To compare the reactogenicity and immunogenicity of an inactivated hepatitis A vaccine in two different immunisation schedules. DESIGN--Randomised trial. SETTING--One London teaching hospital. SUBJECTS--104 healthy adult volunteers (71 men, 33 women aged 19-60). INTERVENTIONS--Hepatitis A vaccine to group 1 (54 volunteers) at 0, 1, and 2 months and to group 2 (50) at 0, 1, and 6 months. MAIN OUTCOME MEASURES--Symptoms at and after each dose; liver function, hepatitis A virus specific serum immune response; and responses in saliva and parotid fluid in immunised volunteers and subjects with natural immunity. RESULTS--The vaccine was well tolerated; 97% (96/99) and 100% of those immunised developed serum antibody after one and two doses of vaccine respectively. Geometric mean titres increased progressively after each dose and were significantly higher in men but not women in group 2 after the third dose (ratio between geometric mean titres 0.265, 95% confidence interval 0.18 to 0.39; p less than 0.001). At one year this group-sex interaction was absent; geometric mean titres for both sexes were significantly higher in group 2 (ratio 0.330, 0.227 to 0.478; p less than 0.0001). Antibody responses were not significantly different between the groups at two years. Compared with naturally infected subjects immunised volunteers developed poor or undetectable virus specific IgG and IgA responses in saliva and parotid fluid. CONCLUSIONS--The vaccine was safe and highly immunogenic, and the differences in the immune responses in saliva and parotid fluid are unlikely to affect its efficacy.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- André F. E., Hepburn A., D'Hondt E. Inactivated candidate vaccines for hepatitis A. Prog Med Virol. 1990;37:72–95. [PubMed] [Google Scholar]
- Chin K. P., Lok A. S., Wong L. S., Lai C. L., Wu P. C. Current seroepidemiology of hepatitis A in Hong Kong. J Med Virol. 1991 Jul;34(3):191–193. doi: 10.1002/jmv.1890340312. [DOI] [PubMed] [Google Scholar]
- Flehmig B., Heinricy U., Pfisterer M. Immunogenicity of a killed hepatitis A vaccine in seronegative volunteers. Lancet. 1989 May 13;1(8646):1039–1041. doi: 10.1016/s0140-6736(89)92443-4. [DOI] [PubMed] [Google Scholar]
- Gruer L. D., McKendrick M. W., Beeching N. J., Geddes A. M. Relapsing hepatitis associated with hepatitis A virus. Lancet. 1982 Jul 17;2(8290):163–163. doi: 10.1016/s0140-6736(82)91136-9. [DOI] [PubMed] [Google Scholar]
- Jacobson I. M., Nath B. J., Dienstag J. L. Relapsing viral hepatitis type A. J Med Virol. 1985 Jun;16(2):163–169. doi: 10.1002/jmv.1890160208. [DOI] [PubMed] [Google Scholar]
- Kani J., Nandwani R., Gilson R. J., Johnson A. M., Maguire H. C., Tedder R. S. Hepatitis A virus infection among homosexual men. BMJ. 1991 Jun 8;302(6789):1399–1399. doi: 10.1136/bmj.302.6789.1399-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpinski K. F., Hayward S., Tryphonas H. Statistical considerations in the quantitation of serum immunoglobulin levels using the enzyme-linked immunosorbent assay (ELISA). J Immunol Methods. 1987 Nov 5;103(2):189–194. doi: 10.1016/0022-1759(87)90289-4. [DOI] [PubMed] [Google Scholar]
- Lim W. L., Yeoh E. K. Hepatitis A vaccination. Lancet. 1992 Feb 1;339(8788):304–304. doi: 10.1016/0140-6736(92)91372-f. [DOI] [PubMed] [Google Scholar]
- Mao J. S. Development of live, attenuated hepatitis A vaccine (H2-strain). Vaccine. 1990 Dec;8(6):523–524. doi: 10.1016/0264-410x(90)90001-3. [DOI] [PubMed] [Google Scholar]
- Parry J. V., Perry K. R., Panday S., Mortimer P. P. Diagnosis of hepatitis A and B by testing saliva. J Med Virol. 1989 Aug;28(4):255–260. doi: 10.1002/jmv.1890280410. [DOI] [PubMed] [Google Scholar]
- Pollock T. M., Reid D. Immunoglobulin for the prevention of infectious hepatitis in persons working overseas. Lancet. 1969 Feb 8;1(7589):281–283. doi: 10.1016/s0140-6736(69)91037-x. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. An inactivated hepatitis A virus vaccine prepared from infected marmoset liver. Proc Soc Exp Biol Med. 1978 Nov;159(2):201–203. doi: 10.3181/00379727-159-40314. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- Stapleton J. T., Jansen R., Lemon S. M. Neutralizing antibody to hepatitis A virus in immune serum globulin and in the sera of human recipients of immune serum globulin. Gastroenterology. 1985 Sep;89(3):637–642. doi: 10.1016/0016-5085(85)90462-7. [DOI] [PubMed] [Google Scholar]
- Tilzey A. J., Banatvala J. E. Hepatitis A. BMJ. 1991 Jun 29;302(6792):1552–1553. doi: 10.1136/bmj.302.6792.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vento S., Garofano T., Di Perri G., Dolci L., Concia E., Bassetti D. Identification of hepatitis A virus as a trigger for autoimmune chronic hepatitis type 1 in susceptible individuals. Lancet. 1991 May 18;337(8751):1183–1187. doi: 10.1016/0140-6736(91)92858-y. [DOI] [PubMed] [Google Scholar]
- Wiedermann G., Ambrosch F., Kollaritsch H., Hofmann H., Kunz C., D'Hondt E., Delem A., André F. E., Safary A., Stéphenne J. Safety and immunogenicity of an inactivated hepatitis A candidate vaccine in healthy adult volunteers. Vaccine. 1990 Dec;8(6):581–584. doi: 10.1016/0264-410x(90)90013-c. [DOI] [PubMed] [Google Scholar]



