Abstract
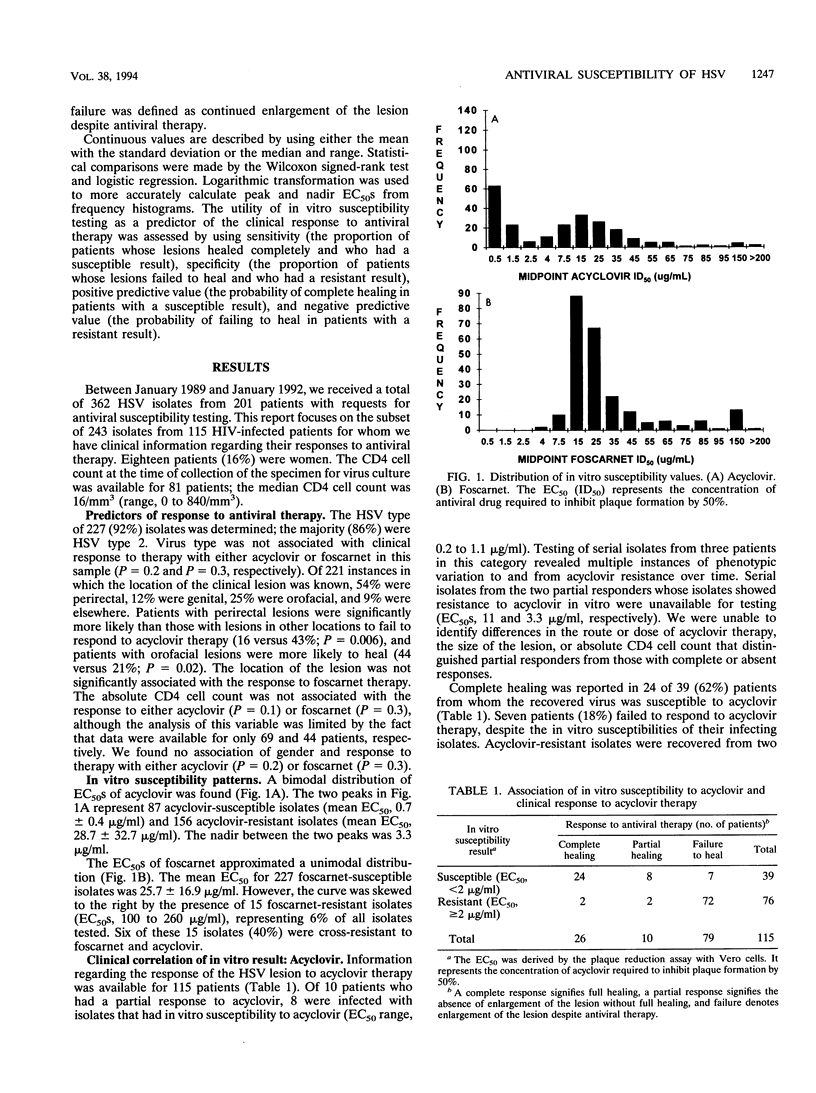
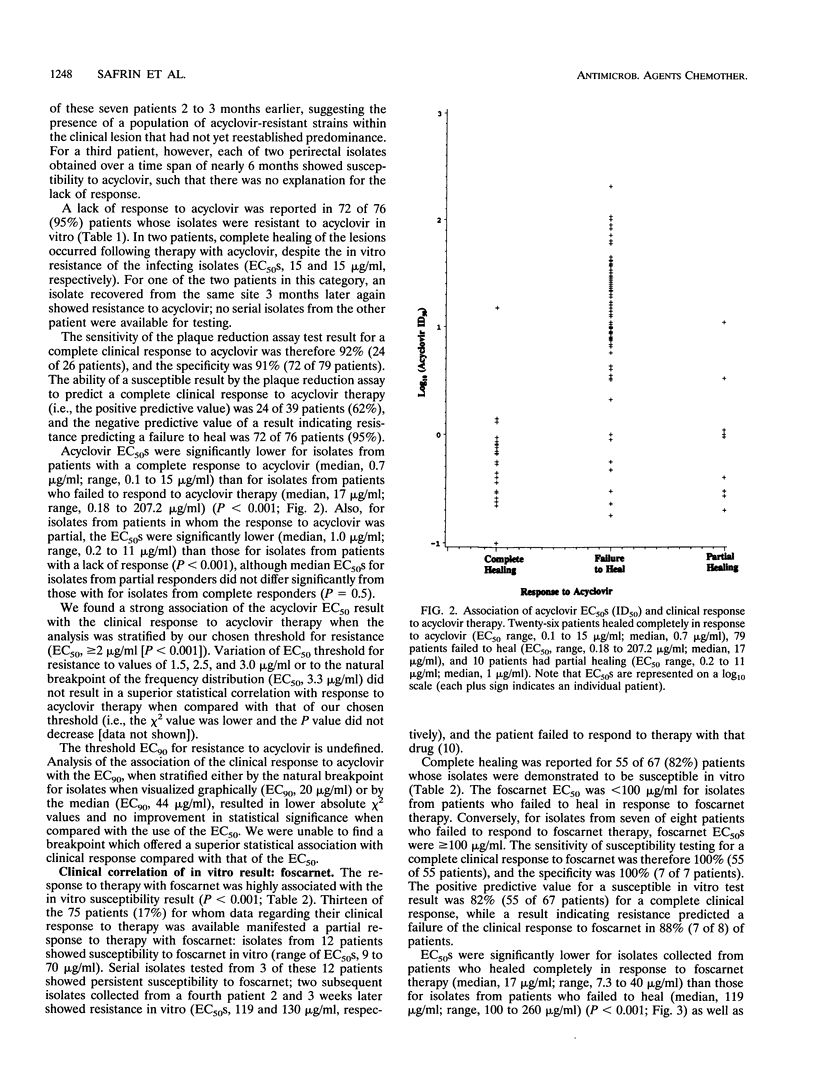
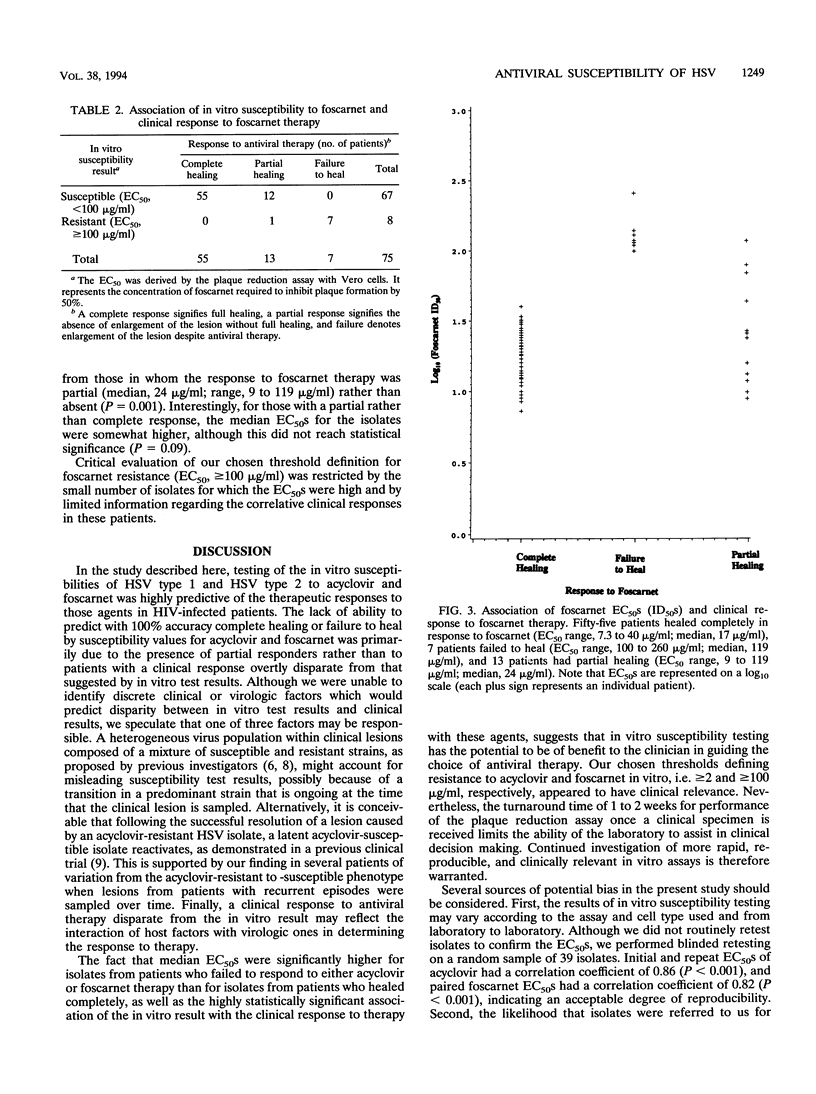
In vitro susceptibility testing of herpes simplex virus (HSV) isolates will play an increasingly important role in guiding the clinical management of immunocompromised hosts who have lesions that are poorly responsive to therapy with standard antiviral agents. We assessed the correlation between the in vitro susceptibility result using a plaque reduction assay in Vero cells and the response to antiviral therapy with acyclovir or foscarnet for 243 clinical isolates of HSV collected from 115 human immunodeficiency virus-infected patients. The in vitro results and clinical responses were highly associated for both acyclovir and foscarnet (P < 0.001 and P < 0.001, respectively). The predictive values of a susceptible result (50% effective concentrations, < 2 micrograms/ml for acyclovir and < 100 micrograms/ml for foscarnet) for complete healing of lesions were 62% for acyclovir and 82% for foscarnet; the predictive values of a resistant result for failure to heal were 95% for acyclovir and 88% for foscarnet. Thus, in vitro testing has clinical utility in guiding therapy, although the 1 to 2 weeks required to derive a definitive result by the plaque reduction assay is a major limitation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Englund J. A., Zimmerman M. E., Swierkosz E. M., Goodman J. L., Scholl D. R., Balfour H. H., Jr Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med. 1990 Mar 15;112(6):416–422. doi: 10.7326/0003-4819-76-3-112-6-416. [DOI] [PubMed] [Google Scholar]
- Erlich K. S., Mills J., Chatis P., Mertz G. J., Busch D. F., Follansbee S. E., Grant R. M., Crumpacker C. S. Acyclovir-resistant herpes simplex virus infections in patients with the acquired immunodeficiency syndrome. N Engl J Med. 1989 Feb 2;320(5):293–296. doi: 10.1056/NEJM198902023200506. [DOI] [PubMed] [Google Scholar]
- McLaren C., Corey L., Dekket C., Barry D. W. In vitro sensitivity to acyclovir in genital herpes simplex viruses from acyclovir-treated patients. J Infect Dis. 1983 Nov;148(5):868–875. doi: 10.1093/infdis/148.5.868. [DOI] [PubMed] [Google Scholar]
- Mertz G. J., Jones C. C., Mills J., Fife K. H., Lemon S. M., Stapleton J. T., Hill E. L., Davis L. G. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. A multicenter double-blind trial. JAMA. 1988 Jul 8;260(2):201–206. [PubMed] [Google Scholar]
- Nilsen A. E., Aasen T., Halsos A. M., Kinge B. R., Tjøtta E. A., Wikström K., Fiddian A. P. Efficacy of oral acyclovir in the treatment of initial and recurrent genital herpes. Lancet. 1982 Sep 11;2(8298):571–573. doi: 10.1016/s0140-6736(82)90658-4. [DOI] [PubMed] [Google Scholar]
- Parris D. S., Harrington J. E. Herpes simplex virus variants restraint to high concentrations of acyclovir exist in clinical isolates. Antimicrob Agents Chemother. 1982 Jul;22(1):71–77. doi: 10.1128/aac.22.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rompalo A. M., Mertz G. J., Davis L. G., Benedetti J., Critchlow C., Stamm W. E., Corey L. Oral acyclovir for treatment of first-episode herpes simplex virus proctitis. JAMA. 1988 May 20;259(19):2879–2881. [PubMed] [Google Scholar]
- Sacks S. L., Wanklin R. J., Reece D. E., Hicks K. A., Tyler K. L., Coen D. M. Progressive esophagitis from acyclovir-resistant herpes simplex. Clinical roles for DNA polymerase mutants and viral heterogeneity? Ann Intern Med. 1989 Dec 1;111(11):893–899. doi: 10.7326/0003-4819-111-11-893. [DOI] [PubMed] [Google Scholar]
- Safrin S., Crumpacker C., Chatis P., Davis R., Hafner R., Rush J., Kessler H. A., Landry B., Mills J. A controlled trial comparing foscarnet with vidarabine for acyclovir-resistant mucocutaneous herpes simplex in the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1991 Aug 22;325(8):551–555. doi: 10.1056/NEJM199108223250805. [DOI] [PubMed] [Google Scholar]
- Safrin S., Kemmerly S., Plotkin B., Smith T., Weissbach N., De Veranez D., Phan L. D., Cohn D. Foscarnet-resistant herpes simplex virus infection in patients with AIDS. J Infect Dis. 1994 Jan;169(1):193–196. doi: 10.1093/infdis/169.1.193. [DOI] [PubMed] [Google Scholar]
- Smith D. W., Goodwin C. S. The use of in-vitro sensitivity testing to predict clinical response of recurrent herpes simplex to suppressive oral acyclovir. J Antimicrob Chemother. 1988 May;21(5):657–664. doi: 10.1093/jac/21.5.657. [DOI] [PubMed] [Google Scholar]


