Abstract
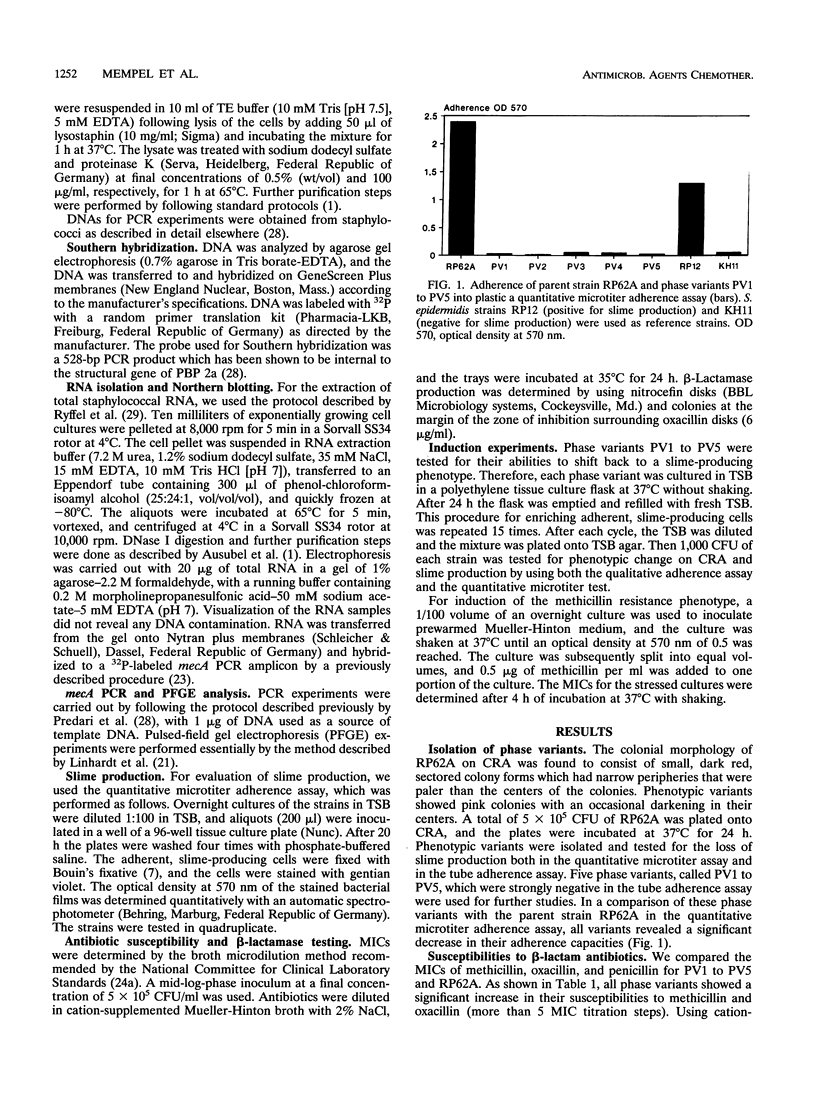
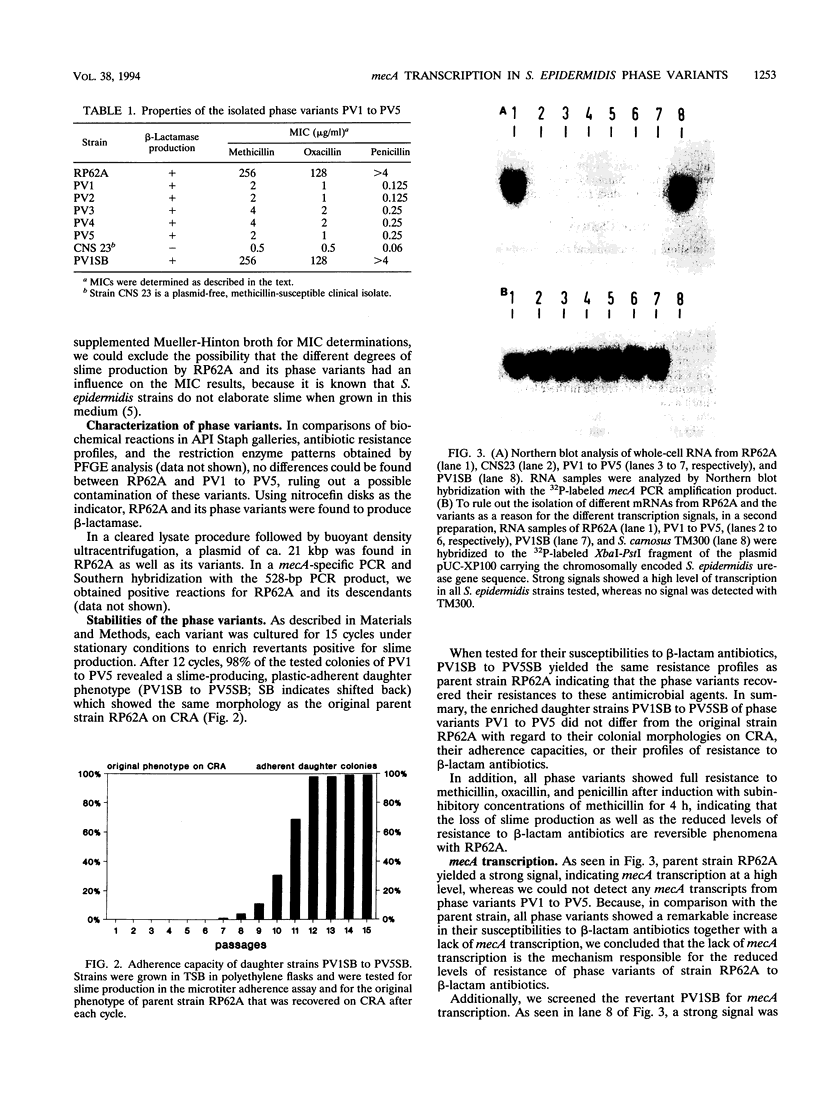
Five phase variants (PV1 to PV5) of the well-characterized, slime-producing, methicillin-resistant, pathogenic strain of Staphylococcus epidermidis sensu strictu RP62A (ATCC 35984) were isolated by the Congo red agar method. In comparison with the parent strain, the phase variants showed a different colonial morphology on Congo red agar, a strongly reduced adherence capacity, and decreased levels of resistance to methicillin, oxacillin, and penicillin. All phase variants yielded biochemical reaction patterns and profiles in pulsed-field gel electrophoresis identical to those of parent strain RP62A, indicating a common origin. All phase variants proved to have the capacity to shift back to the original phenotype of parent strain RP62A. A search for the resistance mechanisms of strain RP62A revealed beta-lactamase production and the presence of mecA in PV1 to PV5 as well as parent strain RP62A. In Northern blots of total staphylococcal RNA, the phase variants showed no detectable mecA-specific transcription product, whereas parent strain RP62A revealed a strong signal, indicating that mecA transcription is not the mechanism responsible for the decreased methicillin resistance phenotype of phase variants PV1 to PV5.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baddour L. M., Barker L. P., Christensen G. D., Parisi J. T., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis in infection of transvenous endocardial pacemaker electrodes. J Clin Microbiol. 1990 Apr;28(4):676–679. doi: 10.1128/jcm.28.4.676-679.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Madison B. M., Parisi J. T., Abraham S. N., Hasty D. L., Lowrance J. H., Josephs J. A., Simpson W. A. Colonial morphology of staphylococci on Memphis agar: phase variation of slime production, resistance to beta-lactam antibiotics, and virulence. J Infect Dis. 1990 Jun;161(6):1153–1169. doi: 10.1093/infdis/161.6.1153. [DOI] [PubMed] [Google Scholar]
- Christensen G. D., Baddour L. M., Simpson W. A. Phenotypic variation of Staphylococcus epidermidis slime production in vitro and in vivo. Infect Immun. 1987 Dec;55(12):2870–2877. doi: 10.1128/iai.55.12.2870-2877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982 Jul;37(1):318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Bisno A. L., Beachey E. H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983 Apr;40(1):407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen G. D., Simpson W. A., Younger J. J., Baddour L. M., Barrett F. F., Melton D. M., Beachey E. H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985 Dec;22(6):996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport D. S., Massanari R. M., Pfaller M. A., Bale M. J., Streed S. A., Hierholzer W. J., Jr Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986 Feb;153(2):332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- Deighton M. A., Capstick J., Borland R. A study of phenotypic variation of Staphylococcus epidermidis using Congo red agar. Epidemiol Infect. 1992 Dec;109(3):423–432. doi: 10.1017/s095026880005041x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighton M., Pearson S., Capstick J., Spelman D., Borland R. Phenotypic variation of Staphylococcus epidermidis isolated from a patient with native valve endocarditis. J Clin Microbiol. 1992 Sep;30(9):2385–2390. doi: 10.1128/jcm.30.9.2385-2390.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Mitoma F., Harding G. K., Hoban D. J., Roberts R. S., Low D. E. Clinical significance of a test for slime production in ventriculoperitoneal shunt infections caused by coagulase-negative staphylococci. J Infect Dis. 1987 Oct;156(4):555–560. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- Eisenstein B. I. Phase variation of type 1 fimbriae in Escherichia coli is under transcriptional control. Science. 1981 Oct 16;214(4518):337–339. doi: 10.1126/science.6116279. [DOI] [PubMed] [Google Scholar]
- Farber B. F., Kaplan M. H., Clogston A. G. Staphylococcus epidermidis extracted slime inhibits the antimicrobial action of glycopeptide antibiotics. J Infect Dis. 1990 Jan;161(1):37–40. doi: 10.1093/infdis/161.1.37. [DOI] [PubMed] [Google Scholar]
- Freeman D. J., Falkiner F. R., Keane C. T. New method for detecting slime production by coagulase negative staphylococci. J Clin Pathol. 1989 Aug;42(8):872–874. doi: 10.1136/jcp.42.8.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray E. D., Peters G., Verstegen M., Regelmann W. E. Effect of extracellular slime substance from Staphylococcus epidermidis on the human cellular immune response. Lancet. 1984 Feb 18;1(8373):365–367. doi: 10.1016/s0140-6736(84)90413-6. [DOI] [PubMed] [Google Scholar]
- Grüter L., Endres M., Gatermann S. Cloning and expression of Staphylococcus epidermidis urease gene sequences in Staphylococcus carnosus. FEMS Microbiol Lett. 1992 May 15;72(1):33–35. doi: 10.1016/0378-1097(92)90485-7. [DOI] [PubMed] [Google Scholar]
- Johnson G. M., Lee D. A., Regelmann W. E., Gray E. D., Peters G., Quie P. G. Interference with granulocyte function by Staphylococcus epidermidis slime. Infect Immun. 1986 Oct;54(1):13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen J. H. Mechanisms of methicillin resistance in Staphylococcus aureus and methods for laboratory detection. Infect Control Hosp Epidemiol. 1991 Jan;12(1):14–19. doi: 10.1086/646233. [DOI] [PubMed] [Google Scholar]
- Kotilainen P. Association of coagulase-negative staphylococcal slime production and adherence with the development and outcome of adult septicemias. J Clin Microbiol. 1990 Dec;28(12):2779–2785. doi: 10.1128/jcm.28.12.2779-2785.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linhardt F., Ziebuhr W., Meyer P., Witte W., Hacker J. Pulsed-field gel electrophoresis of genomic restriction fragments as a tool for the epidemiological analysis of Staphylococcus aureus and coagulase-negative staphylococci. FEMS Microbiol Lett. 1992 Aug 15;74(2-3):181–185. doi: 10.1016/0378-1097(92)90426-o. [DOI] [PubMed] [Google Scholar]
- Mack D., Siemssen N., Laufs R. Parallel induction by glucose of adherence and a polysaccharide antigen specific for plastic-adherent Staphylococcus epidermidis: evidence for functional relation to intercellular adhesion. Infect Immun. 1992 May;60(5):2048–2057. doi: 10.1128/iai.60.5.2048-2057.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morfeldt E., Janzon L., Arvidson S., Löfdahl S. Cloning of a chromosomal locus (exp) which regulates the expression of several exoprotein genes in Staphylococcus aureus. Mol Gen Genet. 1988 Mar;211(3):435–440. doi: 10.1007/BF00425697. [DOI] [PubMed] [Google Scholar]
- Noble M. A., Reid P. E., Park C. M., Chan V. Y. Inhibition of human neutrophil bacteriocidal activity by extracellular substance from slime-producing Staphylococcus epidermidis. Diagn Microbiol Infect Dis. 1986 Apr;4(4):335–339. doi: 10.1016/0732-8893(86)90074-x. [DOI] [PubMed] [Google Scholar]
- Peng H. L., Novick R. P., Kreiswirth B., Kornblum J., Schlievert P. Cloning, characterization, and sequencing of an accessory gene regulator (agr) in Staphylococcus aureus. J Bacteriol. 1988 Sep;170(9):4365–4372. doi: 10.1128/jb.170.9.4365-4372.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller M. A., Herwaldt L. A. Laboratory, clinical, and epidemiological aspects of coagulase-negative staphylococci. Clin Microbiol Rev. 1988 Jul;1(3):281–299. doi: 10.1128/cmr.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Predari S. C., Ligozzi M., Fontana R. Genotypic identification of methicillin-resistant coagulase-negative staphylococci by polymerase chain reaction. Antimicrob Agents Chemother. 1991 Dec;35(12):2568–2573. doi: 10.1128/aac.35.12.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryffel C., Kayser F. H., Berger-Bächi B. Correlation between regulation of mecA transcription and expression of methicillin resistance in staphylococci. Antimicrob Agents Chemother. 1992 Jan;36(1):25–31. doi: 10.1128/aac.36.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Bergström S., Robbins K., Barrera O., Corwin D., Koomey J. M. Gene conversion involving the pilin structural gene correlates with pilus+ in equilibrium with pilus- changes in Neisseria gonorrhoeae. Cell. 1986 Oct 24;47(2):267–276. doi: 10.1016/0092-8674(86)90449-6. [DOI] [PubMed] [Google Scholar]
- Tesch W., Ryffel C., Strässle A., Kayser F. H., Berger-Bächi B. Evidence of a novel staphylococcal mec-encoded element (mecR) controlling expression of penicillin-binding protein 2'. Antimicrob Agents Chemother. 1990 Sep;34(9):1703–1706. doi: 10.1128/aac.34.9.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Nachman S., Leaf H. Stable classes of phenotypic expression in methicillin-resistant clinical isolates of staphylococci. Antimicrob Agents Chemother. 1991 Jan;35(1):124–129. doi: 10.1128/aac.35.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis L. B., MacLowry J. D. Clinically significant differences in antibiograms of morphologic variants of blood culture isolates. Diagn Microbiol Infect Dis. 1989 Mar-Apr;12(2):177–179. doi: 10.1016/0732-8893(89)90010-2. [DOI] [PubMed] [Google Scholar]
- Vandenesch F., Projan S. J., Kreiswirth B., Etienne J., Novick R. P. Agr-related sequences in Staphylococcus lugdunensis. FEMS Microbiol Lett. 1993 Jul 15;111(1):115–122. doi: 10.1111/j.1574-6968.1993.tb06370.x. [DOI] [PubMed] [Google Scholar]
- Younger J. J., Christensen G. D., Bartley D. L., Simmons J. C., Barrett F. F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987 Oct;156(4):548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]



