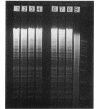
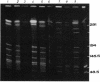
Abstract
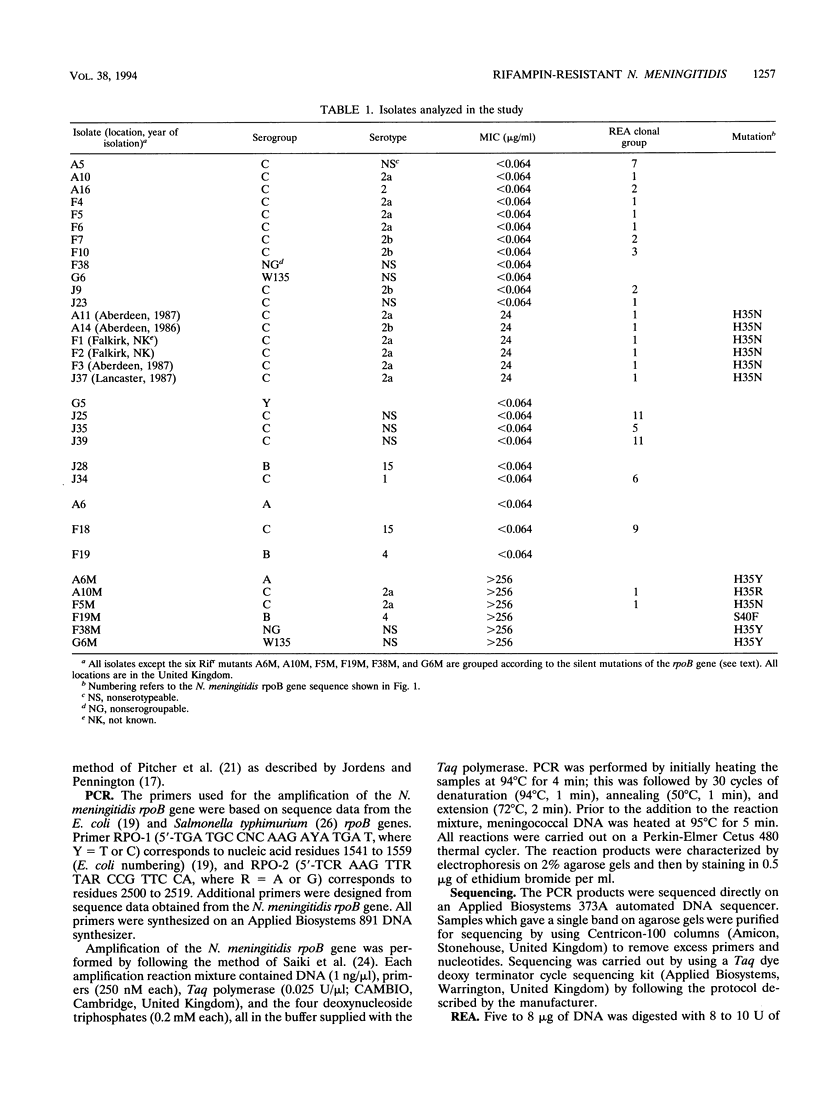
Primers were designed to amplify the rpoB gene of Neisseria meningitidis. The region of the gene amplified covered clusters I and II of the rifampin resistance (Rifr) mutation sites identified in Escherichia coli. DNAs from six Rifr isolates and 21 rifampin-susceptible isolates from the United Kingdom representing a number of serogroups were amplified and sequenced. All six Rifr isolates had identical DNA sequences and the same amino acid change, a His to an Asn change at position 35 (H35N). This His residue is equivalent to the His residue at position 526 in E. coli, one of the known Rifr mutation sites. DNAs from an additional six Rifr mutations generated in vitro were amplified and sequenced. Three had H35Y changes, one had an H35R change, one had an H35N change and one had an S40F change. The predominance of mutations at the His residue at position 35 in Rifr N. meningitidis isolates suggests that it plays a critical role in the selection of antibiotic-resistant variants. All six Rifr isolates belonged to the same clonal group when analyzed by restriction enzyme analysis and pulsed-field gel electrophoresis. These data suggest that a single clone of Rifr N. meningitidis is present and widespread throughout the United Kingdom.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. N., Stocker S. A., Culver D. H., Thornsberry C. Comparison of the E Test to agar dilution, broth microdilution, and agar diffusion susceptibility testing techniques by using a special challenge set of bacteria. J Clin Microbiol. 1991 Mar;29(3):533–538. doi: 10.1128/jcm.29.3.533-538.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bygraves J. A., Maiden M. C. Analysis of the clonal relationships between strains of Neisseria meningitidis by pulsed field gel electrophoresis. J Gen Microbiol. 1992 Mar;138(3):523–531. doi: 10.1099/00221287-138-3-523. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Frøholm L. O., Bøvre K., Holten E., Frasch C. E., Mocca L. F., Zollinger W. D., Selander R. K. Intercontinental spread of a genetically distinctive complex of clones of Neisseria meningitidis causing epidemic disease. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4927–4931. doi: 10.1073/pnas.83.13.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Mocca L. F., Frasch C. E., Frøholm L. O., Zollinger W. D., Selander R. K. Genetic structure of Neisseria meningitidis populations in relation to serogroup, serotype, and outer membrane protein pattern. J Bacteriol. 1987 Jun;169(6):2781–2792. doi: 10.1128/jb.169.6.2781-2792.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deal W. B., Sanders E. Efficacy of rifampin in treatment of meningococcal carriers. N Engl J Med. 1969 Sep 18;281(12):641–645. doi: 10.1056/NEJM196909182811203. [DOI] [PubMed] [Google Scholar]
- Drew T. M., Altman R., Black K., Goldfield M. Minocycline for prophylaxis of infection with Neisseria meningitidis: high rate of side effects in recipients. J Infect Dis. 1976 Feb;133(2):194–198. doi: 10.1093/infdis/133.2.194. [DOI] [PubMed] [Google Scholar]
- Eickhoff T. C. In-vitro and in-vivo studies of resistance to rifampin in meningococci. J Infect Dis. 1971 Apr;123(4):414–420. doi: 10.1093/infdis/123.4.414. [DOI] [PubMed] [Google Scholar]
- Guttler R. B., Counts G. W., Avent C. K., Beaty H. N. Effect of rifampin and minocycline on meningococcal carrier rates. J Infect Dis. 1971 Aug;124(2):199–205. doi: 10.1093/infdis/124.2.199. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Honore N., Cole S. T. Molecular basis of rifampin resistance in Mycobacterium leprae. Antimicrob Agents Chemother. 1993 Mar;37(3):414–418. doi: 10.1128/aac.37.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. J., Cashel M., Friedman D. I., Nakamura Y., Walter W. A., Gross C. A. Effects of rifampicin resistant rpoB mutations on antitermination and interaction with nusA in Escherichia coli. J Mol Biol. 1988 Nov 20;204(2):247–261. doi: 10.1016/0022-2836(88)90573-6. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Characterization of the pleiotropic phenotypes of rifampin-resistant rpoB mutants of Escherichia coli. J Bacteriol. 1989 Sep;171(9):5229–5231. doi: 10.1128/jb.171.9.5229-5231.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. J., Gross C. A. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance. J Mol Biol. 1988 Jul 5;202(1):45–58. doi: 10.1016/0022-2836(88)90517-7. [DOI] [PubMed] [Google Scholar]
- Jin D. J., Walter W. A., Gross C. A. Characterization of the termination phenotypes of rifampicin-resistant mutants. J Mol Biol. 1988 Jul 20;202(2):245–253. doi: 10.1016/0022-2836(88)90455-x. [DOI] [PubMed] [Google Scholar]
- Jones D. M., Kaczmarski E. B. Meningococcal infections in England and Wales: 1992. Commun Dis Rep CDR Rev. 1993 Aug 13;3(9):R129–R131. [PubMed] [Google Scholar]
- Jordens J. Z., Pennington T. H. Characterization of Neisseria meningitidis isolated by ribosomal RNA gene restriction patterns and restriction endonuclease digestion of chromosomal DNA. Epidemiol Infect. 1991 Oct;107(2):253–262. doi: 10.1017/s0950268800048901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Monastyrskaya G. S., Gubanov V. V., Lipkin V. M., Sverdlov E. D., Kiver I. F., Bass I. A., Mindlin S. Z., Danilevskaya O. N., Khesin R. B. Primary structure of Escherichia coli RNA polymerase nucleotide substitution in the beta subunit gene of the rifampicin resistant rpoB255 mutant. Mol Gen Genet. 1981;184(3):536–538. doi: 10.1007/BF00352535. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Monastyrskaya G. S., Gubanov V. V., Guryev S. O., Chertov OYu, Modyanov N. N., Grinkevich V. A., Makarova I. A., Marchenko T. V., Polovnikova I. N. The primary structure of Escherichia coli RNA polymerase. Nucleotide sequence of the rpoB gene and amino-acid sequence of the beta-subunit. Eur J Biochem. 1981 Jun 1;116(3):621–629. doi: 10.1111/j.1432-1033.1981.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Poh C. L., Lau Q. C. Subtyping of Neisseria gonorrhoeae auxotype-serovar groups by pulsed-field gel electrophoresis. J Med Microbiol. 1993 May;38(5):366–370. doi: 10.1099/00222615-38-5-366. [DOI] [PubMed] [Google Scholar]
- Poolman J. T., Lind I., Jónsdóttir K., Frøholm L. O., Jones D. M., Zanen H. C. Meningococcal serotypes and serogroup B disease in north-west Europe. Lancet. 1986 Sep 6;2(8506):555–558. doi: 10.1016/s0140-6736(86)90123-6. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salvi R. J., Ahroon W., Saunders S. S., Arnold S. A. Evoked potentials: computer-automated threshold-tracking procedure using an objective detection criterion. Ear Hear. 1987 Jun;8(3):151–156. [PubMed] [Google Scholar]
- Severinov K., Soushko M., Goldfarb A., Nikiforov V. Rifampicin region revisited. New rifampicin-resistant and streptolydigin-resistant mutants in the beta subunit of Escherichia coli RNA polymerase. J Biol Chem. 1993 Jul 15;268(20):14820–14825. [PubMed] [Google Scholar]
- Sverdlov E. D., Lisitsyn N. A., Gur'ev S. O., Monastyrskaia G. S. Nukleotidnaia posledovatel'nost' rpoB gena Salmonella typhimurium, kodiruiushchego beta-sub''edinitsu RNK-polimerazy. Dokl Akad Nauk SSSR. 1986 Mar-Apr;287(1):232–236. [PubMed] [Google Scholar]
- Telenti A., Imboden P., Marchesi F., Lowrie D., Cole S., Colston M. J., Matter L., Schopfer K., Bodmer T. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet. 1993 Mar 13;341(8846):647–650. doi: 10.1016/0140-6736(93)90417-f. [DOI] [PubMed] [Google Scholar]
- Yagupsky P., Ashkenazi S., Block C. Rifampicin-resistant meningococci causing invasive disease and failure of chemoprophylaxis. Lancet. 1993 May 1;341(8853):1152–1153. doi: 10.1016/0140-6736(93)93171-v. [DOI] [PubMed] [Google Scholar]