Abstract
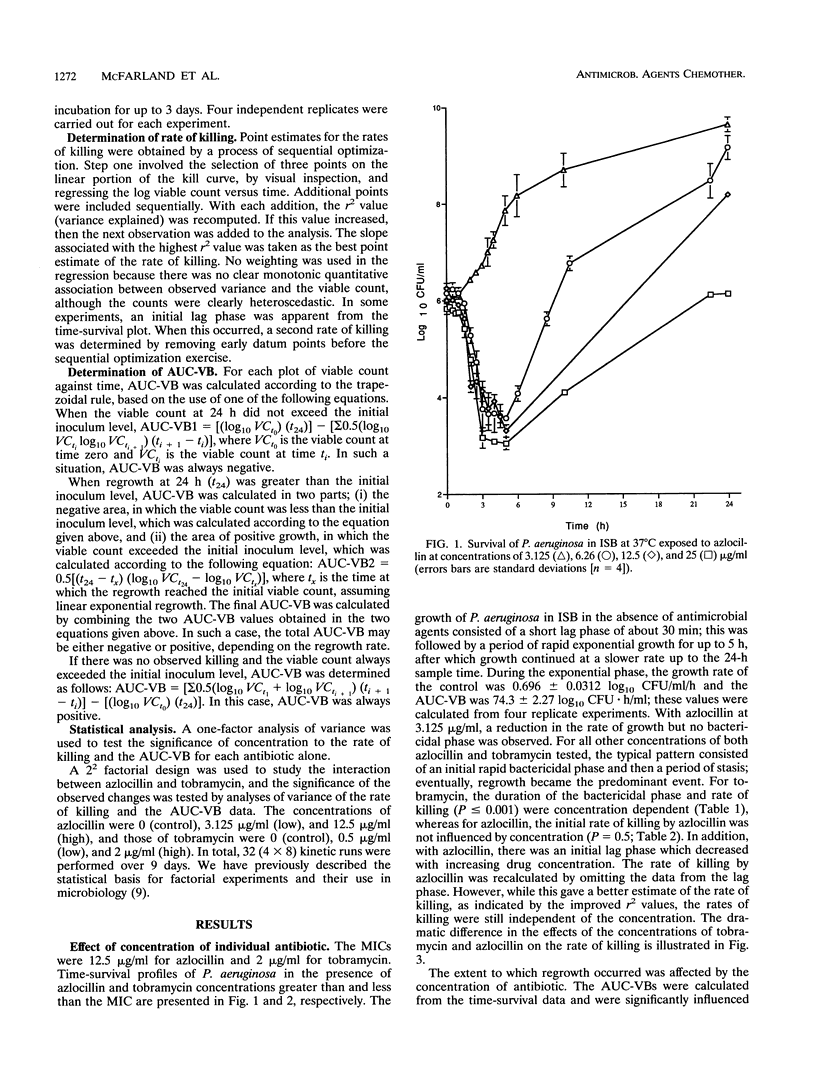
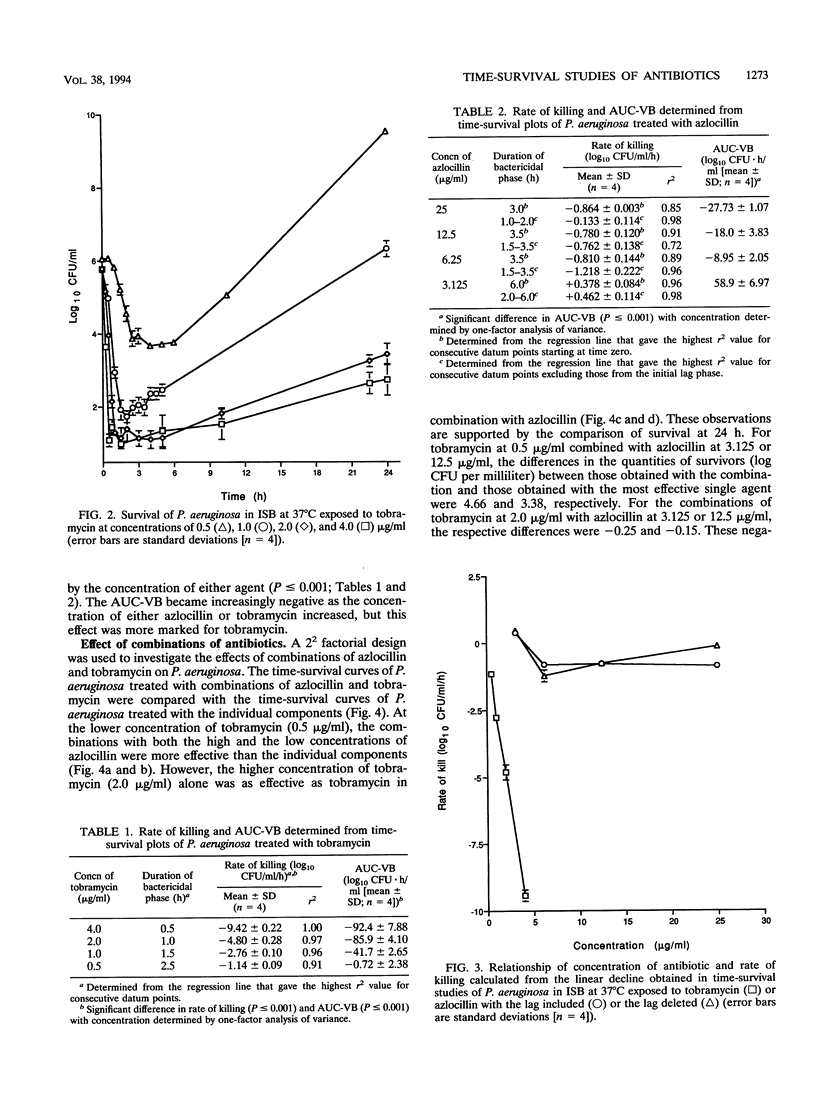
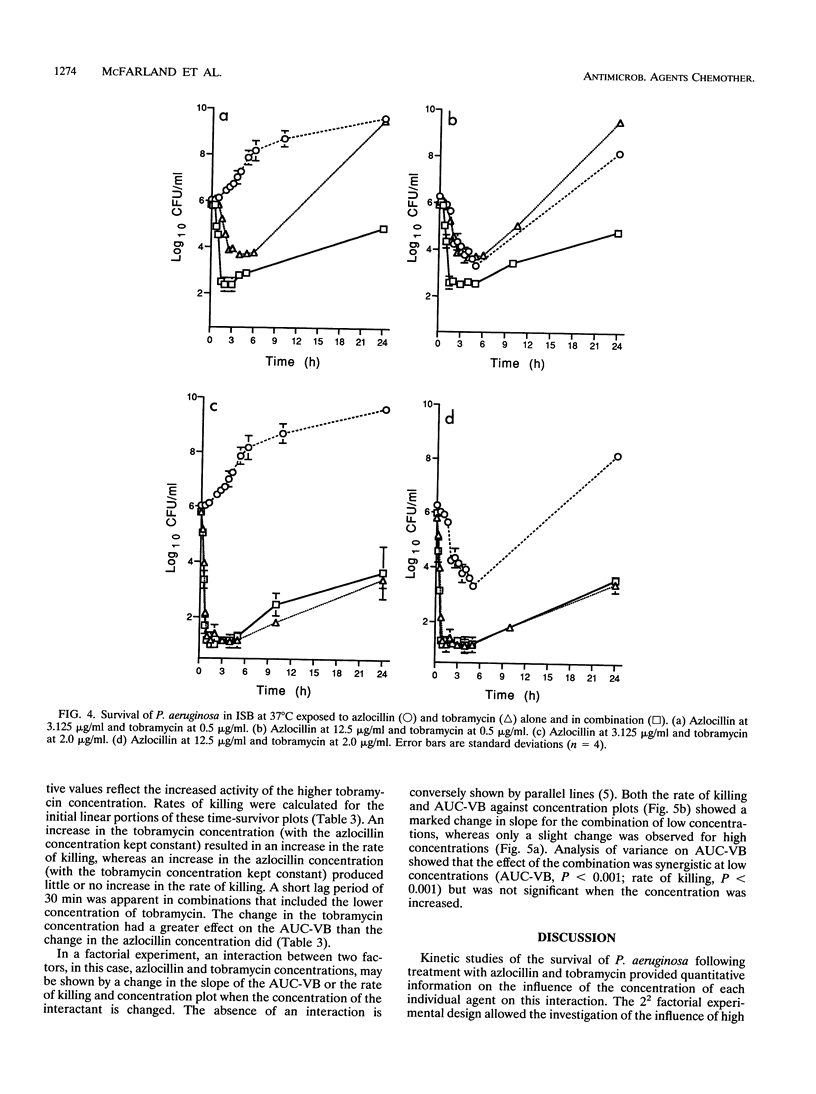
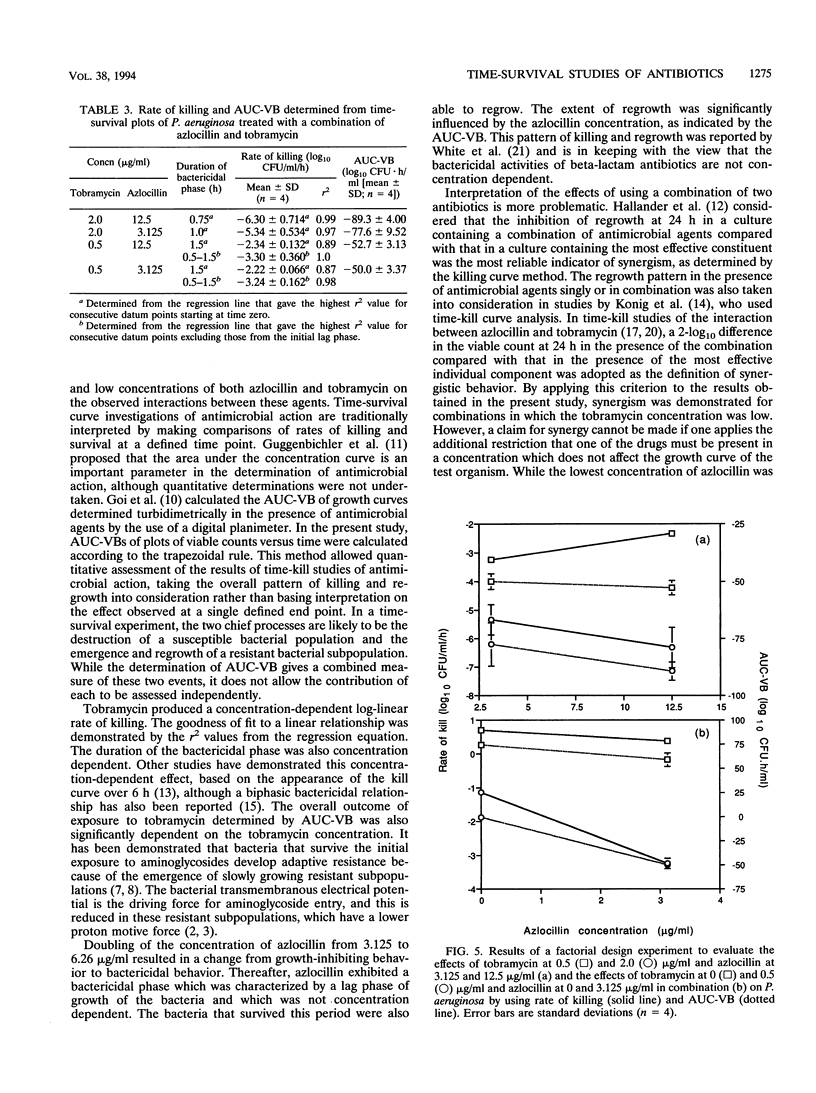
Time-survival studies were conducted to estimate the effects of azlocillin and tobramycin on Pseudomonas aeruginosa NCIMB 8295 (in the exponential phase of growth) at concentrations ranging from one-quarter to twice the MIC. The effects of the individual agents and their combinations were determined by measuring the viable counts (CFU per milliliter) over a 24-h period. The typical pattern observed from the plot of the logarithm of the CFU per milliliter against time was an initial rapid killing; this was followed by a period of stasis and regrowth. Initial rates of killing by tobramycin were concentration dependent, whereas this was not the case with azlocillin. From the time-survivor plots, the area under the curve for viable bacteria was also calculated. It offered a useful method of interpreting the results of time-kill studies, taking the overall pattern of killing and regrowth into consideration. The area under the curve for viable bacteria was concentration dependent for both antibiotics. A 2(2) factorial experimental design was used to analyze the joint effects of azlocillin and tobramycin. In such a factorial experiment, an interaction between two factors, in this case, azlocillin and tobramycin concentrations, is shown by a change in the slope of the plot when the concentration of the interactant is changed. Analysis of variance showed that the combination was synergistic at low concentrations, but this was not significant when the concentration of either interactant was increased.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bakker-Woudenberg I. A., Roosendaal R. Impact of dosage regimens on the efficacy of antibiotics in the immunocompromised host. J Antimicrob Chemother. 1988 Feb;21(2):145–147. doi: 10.1093/jac/21.2.145. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Mechanisms of aminoglycoside resistance of anaerobic bacteria and facultative bacteria grown anaerobically. J Antimicrob Chemother. 1981 Dec;8 (Suppl 500):1–8. doi: 10.1093/jac/8.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- Bryan L. E., Kwan S. Roles of ribosomal binding, membrane potential, and electron transport in bacterial uptake of streptomycin and gentamicin. Antimicrob Agents Chemother. 1983 Jun;23(6):835–845. doi: 10.1128/aac.23.6.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin N. X., Neu H. C. Synergy of azlocillin with aminoglycosides. J Antimicrob Chemother. 1983 May;11 (Suppl B):33–38. doi: 10.1093/jac/11.suppl_b.33. [DOI] [PubMed] [Google Scholar]
- Daikos G. L., Lolans V. T., Jackson G. G. First-exposure adaptive resistance to aminoglycoside antibiotics in vivo with meaning for optimal clinical use. Antimicrob Agents Chemother. 1991 Jan;35(1):117–123. doi: 10.1128/aac.35.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerber A. U., Craig W. A. Aminoglycoside-selected subpopulations of Pseudomonas aeruginosa: characterization and virulence in normal and leukopenic mice. J Lab Clin Med. 1982 Nov;100(5):671–681. [PubMed] [Google Scholar]
- Gerber A. U., Vastola A. P., Brandel J., Craig W. A. Selection of aminoglycoside-resistant variants of Pseudomonas aeruginosa in an in vivo model. J Infect Dis. 1982 Nov;146(5):691–697. doi: 10.1093/infdis/146.5.691. [DOI] [PubMed] [Google Scholar]
- Gilliland D., Li Wan Po A., Scott E. Kinetic evaluation of claimed synergistic paraben combinations using a factorial design. J Appl Bacteriol. 1992 Mar;72(3):258–261. doi: 10.1111/j.1365-2672.1992.tb01832.x. [DOI] [PubMed] [Google Scholar]
- Goi H., Inouye S., Kitasato I. Bacteriolytic combination effect of cefminox and piperacillin evaluated by turbidimetry. Drugs Exp Clin Res. 1989;15(9):397–407. [PubMed] [Google Scholar]
- Guggenbichler J. P., Semenitz E., König P. Kill kinetics and regrowth pattern of bacteria exposed to antibiotic concentrations simulating those observed in vivo. J Antimicrob Chemother. 1985 Jan;15 (Suppl A):139–146. doi: 10.1093/jac/15.suppl_a.139. [DOI] [PubMed] [Google Scholar]
- Hallander H. O., Dornbusch K., Gezelius L., Jacobson K., Karlsson I. Synergism between aminoglycosides and cephalosporins with antipseudomonal activity: interaction index and killing curve method. Antimicrob Agents Chemother. 1982 Nov;22(5):743–752. doi: 10.1128/aac.22.5.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapusnik J. E., Hackbarth C. J., Chambers H. F., Carpenter T., Sande M. A. Single, large, daily dosing versus intermittent dosing of tobramycin for treating experimental pseudomonas pneumonia. J Infect Dis. 1988 Jul;158(1):7–12. doi: 10.1093/infdis/158.1.7. [DOI] [PubMed] [Google Scholar]
- König P., Guggenbichler J. P., Semenitz E., Foisner W. Kill kinetics of bacteria under fluctuating concentrations of various antibiotics. II. Description of experiments. Chemotherapy. 1986;32(1):44–58. doi: 10.1159/000238388. [DOI] [PubMed] [Google Scholar]
- MacArthur R. D., Lolans V., Zar F. A., Jackson G. G. Biphasic, concentration-dependent and rate-limited, concentration-independent bacterial killing by an aminoglycoside antibiotic. J Infect Dis. 1984 Nov;150(5):778–779. doi: 10.1093/infdis/150.5.778. [DOI] [PubMed] [Google Scholar]
- Marks M. I. Antibiotic therapy for bronchopulmonary infections in cystic fibrosis. The American approach. Antibiot Chemother (1971) 1989;42:229–236. doi: 10.1159/000417624. [DOI] [PubMed] [Google Scholar]
- Norden C. W., Shaffer M. A. Activities of tobramycin and azlocillin alone and in combination against experimental osteomyelitis caused by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1982 Jan;21(1):62–65. doi: 10.1128/aac.21.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. E., Davey P. G. Practicalities of once-daily aminoglycoside dosing. J Antimicrob Chemother. 1993 Jan;31(1):4–8. doi: 10.1093/jac/31.1.4. [DOI] [PubMed] [Google Scholar]
- Reyes M. P., Smith F., Lerner A. M. Studies of in vitro synergy between several beta-lactam and aminoglycoside antibiotics against endocarditis strains of Pseudomonas aeruginosa. J Infect. 1984 Mar;8(2):110–117. doi: 10.1016/s0163-4453(84)92385-5. [DOI] [PubMed] [Google Scholar]
- Watanakunakorn C. Effects of inoculum size on the activity of carboxy- and ureido-penicillins and effects of combinations of ureido-penicillins with aminoglycosides against resistant Pseudomonas aeruginosa. J Antimicrob Chemother. 1986 Jan;17(1):91–95. doi: 10.1093/jac/17.1.91. [DOI] [PubMed] [Google Scholar]
- White A. R., Comber K. R., Sutherland R. Comparative bactericidal effects of azlocillin and ticarcillin against Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1980 Jul;18(1):182–189. doi: 10.1128/aac.18.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]


