Abstract
Wild-type mutants highly resistant to fluoroquinolones were selected in vitro from a quinolone-susceptible Escherichia coli isolate by stepwise exposure to increasing concentrations of nalidixic acid and ciprofloxacin (CIP) either in liquid medium or on solid medium. Mutant R17 was selected by serial passage in liquid medium; the MIC of CIP for mutant R17 was 256 micrograms/ml. On solid medium, consecutive mutants MI, MII, MIII, MIVa, and MIVb were selected in four steps. The frequencies of mutations were between 10(-9) and 10(-11), and the MICs of CIP ranged from 0.5 microgram/ml (for mutant MI) to 256 micrograms/ml (for mutant MIVb). From the results of a dominance test with the gyrB+ plasmid (pBP547), no gyrB mutations were detectable. In the first step, mutant MI, a mutation from a Ser to a Leu residue at position 83 (a Ser-83-->Leu mutation), was detected in the quinolone resistance-determining region of the gyrA gene. In addition, the second-step mutation was associated with a reduced uptake of CIP and an altered outer membrane protein profile. The third mutation was identified as an Asp-87-->Gly mutation in the quinolone resistance-determining region of the gyrA gene. Concomitantly, a slight increase in the doubling time was detected. For two different four-step mutants, mutants MIVa and MIVb, the MICs of only some quinolones, including CIP, increased. The accumulation of CIP in the mutants was comparable to that in their parent MIII. The doubling time of mutant MIVa was similar to that of mutant MIII, but differed by a factor of 3 from that of the very slow growing mutant MIVb. In contrast, a clinical isolate of E.coli (isolate 205096) described previously (P. Heisig, H. Schedletzky, and H. Falkenstein-Paul, Antimicrob. Agents Chemother. 37:696-701, 1993) which has the same double mutation in gyrA had a doubling time comparable to that of the wild-type isolate.
Full text
PDF

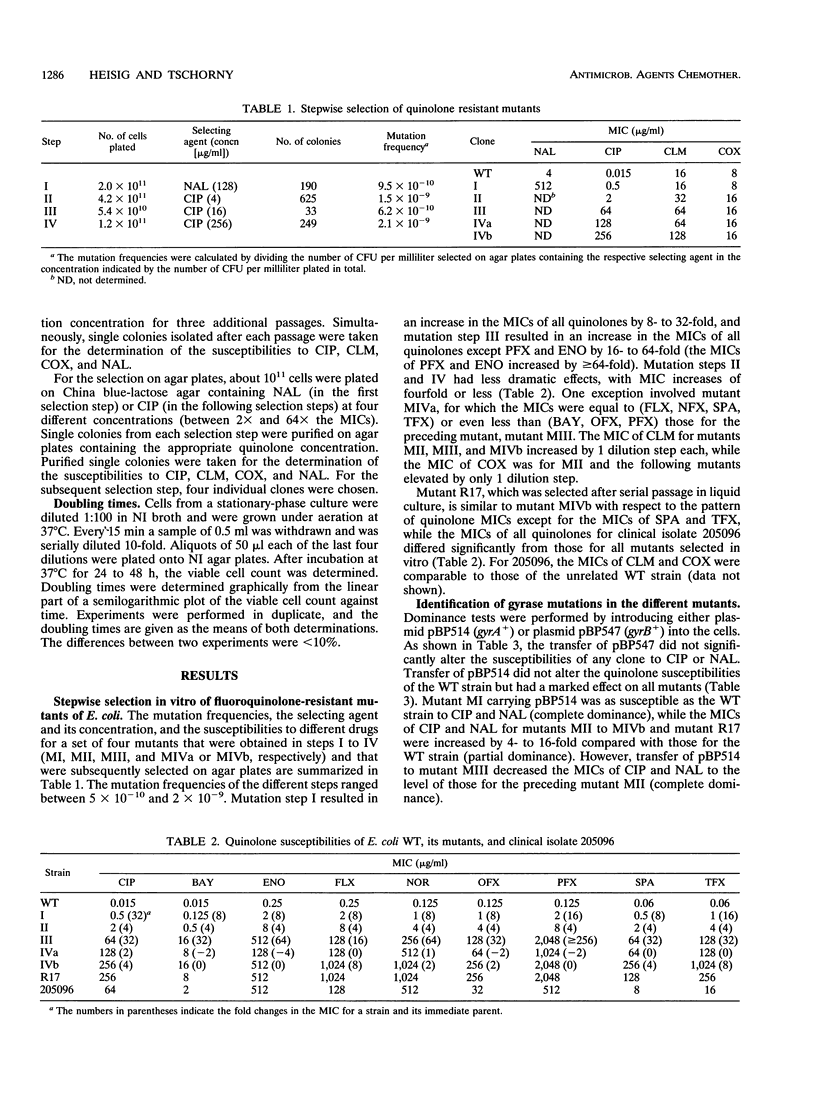

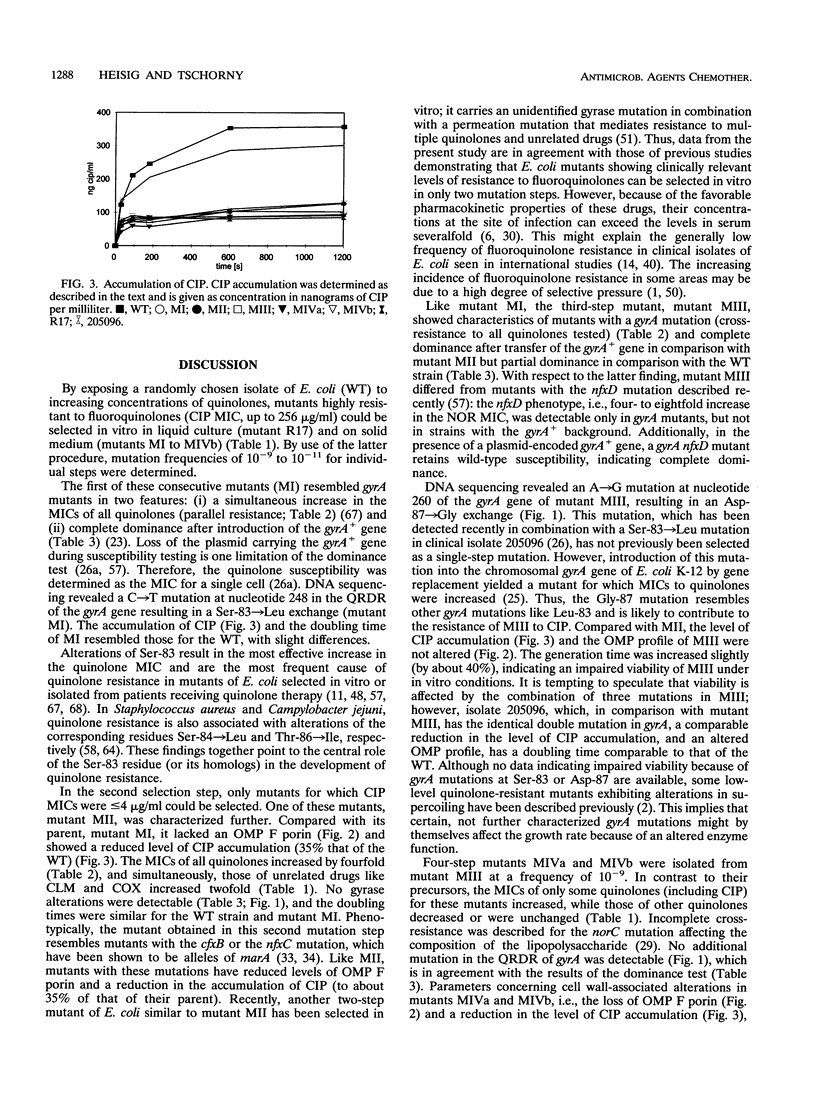



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguiar J. M., Chacon J., Canton R., Baquero F. The emergence of highly fluoroquinolone-resistant Escherichia coli in community-acquired urinary tract infections. J Antimicrob Chemother. 1992 Mar;29(3):349–350. doi: 10.1093/jac/29.3.349. [DOI] [PubMed] [Google Scholar]
- Aleixandre V., Urios A., Herrera G., Blanco M. New Escherichia coli gyrA and gyrB mutations which have a graded effect on DNA supercoiling. Mol Gen Genet. 1989 Oct;219(1-2):306–312. doi: 10.1007/BF00261192. [DOI] [PubMed] [Google Scholar]
- Aoyama H., Sato K., Kato T., Hirai K., Mitsuhashi S. Norfloxacin resistance in a clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1987 Oct;31(10):1640–1641. doi: 10.1128/aac.31.10.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellido F., Pechère J. C. Laboratory survey of fluoroquinolone activity. Rev Infect Dis. 1989 Jul-Aug;11 (Suppl 5):S917–S924. doi: 10.1093/clinids/11.supplement_5.s917. [DOI] [PubMed] [Google Scholar]
- Brismar B., Edlund C., Malmborg A. S., Nord C. E. Ciprofloxacin concentrations and impact of the colon microflora in patients undergoing colorectal surgery. Antimicrob Agents Chemother. 1990 Mar;34(3):481–483. doi: 10.1128/aac.34.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cambau E., Bordon F., Collatz E., Gutmann L. Novel gyrA point mutation in a strain of Escherichia coli resistant to fluoroquinolones but not to nalidixic acid. Antimicrob Agents Chemother. 1993 Jun;37(6):1247–1252. doi: 10.1128/aac.37.6.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai T. J., Foulds J. Escherichia coli K-12 tolF mutants: alterations in protein composition of the outer membrane. J Bacteriol. 1977 May;130(2):781–786. doi: 10.1128/jb.130.2.781-786.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J. S., Georgopapadakou N. H. Fluorometric assay for fleroxacin uptake by bacterial cells. Antimicrob Agents Chemother. 1989 Jan;33(1):27–29. doi: 10.1128/aac.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. P., Hooper D. C., Wolfson J. S., Souza K. S., McMurry L. M., Levy S. B. Endogenous active efflux of norfloxacin in susceptible Escherichia coli. Antimicrob Agents Chemother. 1988 Aug;32(8):1187–1191. doi: 10.1128/aac.32.8.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen M. E., Wyke A. W., Kuroda R., Fisher L. M. Cloning and characterization of a DNA gyrase A gene from Escherichia coli that confers clinical resistance to 4-quinolones. Antimicrob Agents Chemother. 1989 Jun;33(6):886–894. doi: 10.1128/aac.33.6.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Dorman C. J., Lynch A. S., Ni Bhriain N., Higgins C. F. DNA supercoiling in Escherichia coli: topA mutations can be suppressed by DNA amplifications involving the tolC locus. Mol Microbiol. 1989 Apr;3(4):531–540. doi: 10.1111/j.1365-2958.1989.tb00199.x. [DOI] [PubMed] [Google Scholar]
- Dornbusch K. Resistance to beta-lactam antibiotics and ciprofloxacin in gram-negative bacilli and staphylococci isolated from blood: a European collaborative study. European Study Group on Antibiotic Resistance. J Antimicrob Chemother. 1990 Aug;26(2):269–278. doi: 10.1093/jac/26.2.269. [DOI] [PubMed] [Google Scholar]
- Dri A. M., Moreau P. L., Rouvière-Yaniv J. Role of the histone-like proteins OsmZ and HU in homologous recombination. Gene. 1992 Oct 12;120(1):11–16. doi: 10.1016/0378-1119(92)90003-8. [DOI] [PubMed] [Google Scholar]
- Drlica K. Biology of bacterial deoxyribonucleic acid topoisomerases. Microbiol Rev. 1984 Dec;48(4):273–289. doi: 10.1128/mr.48.4.273-289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drlica K. Control of bacterial DNA supercoiling. Mol Microbiol. 1992 Feb;6(4):425–433. doi: 10.1111/j.1365-2958.1992.tb01486.x. [DOI] [PubMed] [Google Scholar]
- Duckworth G. J., Williams J. D. Frequency of appearance of resistant variants to norfloxacin and nalidixic acid. J Antimicrob Chemother. 1984 May;13 (Suppl B):33–38. doi: 10.1093/jac/13.suppl_b.33. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Graeme-Cook K. A., May G., Bremer E., Higgins C. F. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol Microbiol. 1989 Sep;3(9):1287–1294. doi: 10.1111/j.1365-2958.1989.tb00279.x. [DOI] [PubMed] [Google Scholar]
- Hallett P., Maxwell A. Novel quinolone resistance mutations of the Escherichia coli DNA gyrase A protein: enzymatic analysis of the mutant proteins. Antimicrob Agents Chemother. 1991 Feb;35(2):335–340. doi: 10.1128/aac.35.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P., Mehlert A., Maxwell A. Escherichia coli cells resistant to the DNA gyrase inhibitor, ciprofloxacin, overproduce a 60 kD protein homologous to GroEL. Mol Microbiol. 1990 Mar;4(3):345–353. doi: 10.1111/j.1365-2958.1990.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Hane M. W., Wood T. H. Escherichia coli K-12 mutants resistant to nalidixic acid: genetic mapping and dominance studies. J Bacteriol. 1969 Jul;99(1):238–241. doi: 10.1128/jb.99.1.238-241.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisig P. High-level fluoroquinolone resistance in a Salmonella typhimurium isolate due to alterations in both gyrA and gyrB genes. J Antimicrob Chemother. 1993 Sep;32(3):367–377. doi: 10.1093/jac/32.3.367. [DOI] [PubMed] [Google Scholar]
- Heisig P., Schedletzky H., Falkenstein-Paul H. Mutations in the gyrA gene of a highly fluoroquinolone-resistant clinical isolate of Escherichia coli. Antimicrob Agents Chemother. 1993 Apr;37(4):696–701. doi: 10.1128/aac.37.4.696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisig P., Wiedemann B. Use of a broad-host-range gyrA plasmid for genetic characterization of fluoroquinolone-resistant gram-negative bacteria. Antimicrob Agents Chemother. 1991 Oct;35(10):2031–2036. doi: 10.1128/aac.35.10.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins C. F., Dorman C. J., Stirling D. A., Waddell L., Booth I. R., May G., Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988 Feb 26;52(4):569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Irikura T., Iyobe S., Mitsuhashi S. Differences in susceptibility to quinolones of outer membrane mutants of Salmonella typhimurium and Escherichia coli. Antimicrob Agents Chemother. 1986 Mar;29(3):535–538. doi: 10.1128/aac.29.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai K., Aoyama H., Suzue S., Irikura T., Iyobe S., Mitsuhashi S. Isolation and characterization of norfloxacin-resistant mutants of Escherichia coli K-12. Antimicrob Agents Chemother. 1986 Aug;30(2):248–253. doi: 10.1128/aac.30.2.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Ng E. Y., Swartz M. N. Mechanisms of action of and resistance to ciprofloxacin. Am J Med. 1987 Apr 27;82(4A):12–20. [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Ng E. Y., McHugh G. L., Swartz M. N. Mechanisms of quinolone resistance in Escherichia coli: characterization of nfxB and cfxB, two mutant resistance loci decreasing norfloxacin accumulation. Antimicrob Agents Chemother. 1989 Mar;33(3):283–290. doi: 10.1128/aac.33.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S., Souza K. S., Tung C., McHugh G. L., Swartz M. N. Genetic and biochemical characterization of norfloxacin resistance in Escherichia coli. Antimicrob Agents Chemother. 1986 Apr;29(4):639–644. doi: 10.1128/aac.29.4.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper D. C., Wolfson J. S. The fluoroquinolones: pharmacology, clinical uses, and toxicities in humans. Antimicrob Agents Chemother. 1985 Nov;28(5):716–721. doi: 10.1128/aac.28.5.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz D. S., Wang J. C. Mapping the active site tyrosine of Escherichia coli DNA gyrase. J Biol Chem. 1987 Apr 15;262(11):5339–5344. [PubMed] [Google Scholar]
- Hulton C. S., Seirafi A., Hinton J. C., Sidebotham J. M., Waddell L., Pavitt G. D., Owen-Hughes T., Spassky A., Buc H., Higgins C. F. Histone-like protein H1 (H-NS), DNA supercoiling, and gene expression in bacteria. Cell. 1990 Nov 2;63(3):631–642. doi: 10.1016/0092-8674(90)90458-q. [DOI] [PubMed] [Google Scholar]
- Ishii H., Sato K., Hoshino K., Sato M., Yamaguchi A., Sawai T., Osada Y. Active efflux of ofloxacin by a highly quinolone-resistant strain of Proteus vulgaris. J Antimicrob Chemother. 1991 Dec;28(6):827–836. doi: 10.1093/jac/28.6.827. [DOI] [PubMed] [Google Scholar]
- Jonsson M., Walder M., Forsgren A. First clinical isolate of highly fluoroquinolone-resistant Escherichia coli in Scandanavia. Eur J Clin Microbiol Infect Dis. 1990 Nov;9(11):851–853. doi: 10.1007/BF01967392. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Ruble C. A. Efflux-mediated fluoroquinolone resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1993 May;37(5):1086–1094. doi: 10.1128/aac.37.5.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kresken M., Wiedemann B. Development of resistance to nalidixic acid and the fluoroquinolones after the introduction of norfloxacin and ofloxacin. Antimicrob Agents Chemother. 1988 Aug;32(8):1285–1288. doi: 10.1128/aac.32.8.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitsuyama J., Itoh Y., Takahata M., Okamoto S., Yasuda T. In vitro antibacterial activities of tosufloxacin against and uptake of tosufloxacin by outer membrane mutants of Escherichia coli, Proteus mirabilis, and Salmonella typhimurium. Antimicrob Agents Chemother. 1992 Sep;36(9):2030–2036. doi: 10.1128/aac.36.9.2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniot-Ville N., Guibert J., Moreau N., Acar J. F., Collatz E., Gutmann L. Mechanisms of quinolone resistance in a clinical isolate of Escherichia coli highly resistant to fluoroquinolones but susceptible to nalidixic acid. Antimicrob Agents Chemother. 1991 Mar;35(3):519–523. doi: 10.1128/aac.35.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni Bhriain N., Dorman C. J., Higgins C. F. An overlap between osmotic and anaerobic stress responses: a potential role for DNA supercoiling in the coordinate regulation of gene expression. Mol Microbiol. 1989 Jul;3(7):933–942. doi: 10.1111/j.1365-2958.1989.tb00243.x. [DOI] [PubMed] [Google Scholar]
- Ni Bhriain N., Dorman C. J. Isolation and characterization of a topA mutant of Shigella flexneri. Mol Microbiol. 1993 Feb;7(3):351–358. doi: 10.1111/j.1365-2958.1993.tb01127.x. [DOI] [PubMed] [Google Scholar]
- Oram M., Fisher L. M. 4-Quinolone resistance mutations in the DNA gyrase of Escherichia coli clinical isolates identified by using the polymerase chain reaction. Antimicrob Agents Chemother. 1991 Feb;35(2):387–389. doi: 10.1128/aac.35.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram M., Fisher L. M. An Escherichia coli DNA topoisomerase I mutant has a compensatory mutation that alters two residues between functional domains of the DNA gyrase A protein. J Bacteriol. 1992 Jun;174(12):4175–4178. doi: 10.1128/jb.174.12.4175-4178.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piddock L. J., Hall M. C., Walters R. N. Phenotypic characterization of quinolone-resistant mutants of Enterobacteriaceae selected from wild type, gyrA type and multiply-resistant (marA) type strains. J Antimicrob Chemother. 1991 Aug;28(2):185–198. doi: 10.1093/jac/28.2.185. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Traynor E. A., Wise R. A comparison of the mechanisms of decreased susceptibility of aztreonam-resistant and ceftazidime-resistant Enterobacteriaceae. J Antimicrob Chemother. 1990 Dec;26(6):749–762. doi: 10.1093/jac/26.6.749. [DOI] [PubMed] [Google Scholar]
- Piddock L. J., Wise R. Mechanisms of resistance to quinolones and clinical perspectives. J Antimicrob Chemother. 1989 Apr;23(4):475–480. doi: 10.1093/jac/23.4.475. [DOI] [PubMed] [Google Scholar]
- Pérez-Trallero E., Urbieta M., Jimenez D., García-Arenzana J. M., Cilla G. Ten-year survey of quinolone resistance in Escherichia coli causing urinary tract infections. Eur J Clin Microbiol Infect Dis. 1993 May;12(5):349–351. doi: 10.1007/BF01964432. [DOI] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M., Drlica K. DNA gyrase on the bacterial chromosome: DNA cleavage induced by oxolinic acid. J Mol Biol. 1979 Jun 25;131(2):287–302. doi: 10.1016/0022-2836(79)90077-9. [DOI] [PubMed] [Google Scholar]
- Soussy C. J., Wolfson J. S., Ng E. Y., Hooper D. C. Limitations of plasmid complementation test for determination of quinolone resistance due to changes in the gyrase A protein and identification of conditional quinolone resistance locus. Antimicrob Agents Chemother. 1993 Dec;37(12):2588–2592. doi: 10.1128/aac.37.12.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreedharan S., Oram M., Jensen B., Peterson L. R., Fisher L. M. DNA gyrase gyrA mutations in ciprofloxacin-resistant strains of Staphylococcus aureus: close similarity with quinolone resistance mutations in Escherichia coli. J Bacteriol. 1990 Dec;172(12):7260–7262. doi: 10.1128/jb.172.12.7260-7262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanberg S. L., Wang J. C. Cloning and sequencing of the Escherichia coli gyrA gene coding for the A subunit of DNA gyrase. J Mol Biol. 1987 Oct 20;197(4):729–736. doi: 10.1016/0022-2836(87)90479-7. [DOI] [PubMed] [Google Scholar]
- Tenney J. H., Maack R. W., Chippendale G. R. Rapid selection of organisms with increasing resistance on subinhibitory concentrations of norfloxacin in agar. Antimicrob Agents Chemother. 1983 Jan;23(1):188–189. doi: 10.1128/aac.23.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Wang Y., Huang W. M., Taylor D. E. Cloning and nucleotide sequence of the Campylobacter jejuni gyrA gene and characterization of quinolone resistance mutations. Antimicrob Agents Chemother. 1993 Mar;37(3):457–463. doi: 10.1128/aac.37.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi J., Yoshida H., Yamayoshi M., Nakamura S. Nalidixic acid-resistant mutations of the gyrB gene of Escherichia coli. Mol Gen Genet. 1986 Sep;204(3):367–373. doi: 10.1007/BF00331012. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Bogaki M., Nakamura M., Nakamura S. Quinolone resistance-determining region in the DNA gyrase gyrA gene of Escherichia coli. Antimicrob Agents Chemother. 1990 Jun;34(6):1271–1272. doi: 10.1128/aac.34.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida H., Kojima T., Yamagishi J., Nakamura S. Quinolone-resistant mutations of the gyrA gene of Escherichia coli. Mol Gen Genet. 1988 Jan;211(1):1–7. doi: 10.1007/BF00338386. [DOI] [PubMed] [Google Scholar]