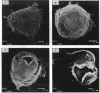
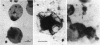
Abstract
The strain Bacillus licheniformis D-13 produces three hydrophobic peptides (amoebicins d13-A, d13-B, and d13-C) that elicit antiamoebic activity against human-pathogenic and nonpathogenic species of Naegleria and have a broad spectrum of antibacterial activity. The three amoebicins have the same amino acid composition (three Asp, two Glu, two Val, and nine Leu residues) and molecular weight (1,870). Amoebicin d13-B causes lysis of amoebae through disorganization of the cell membrane. It also induces permeability to 86Rb and membrane disruption in asolectin vesicles.
Full text
PDF
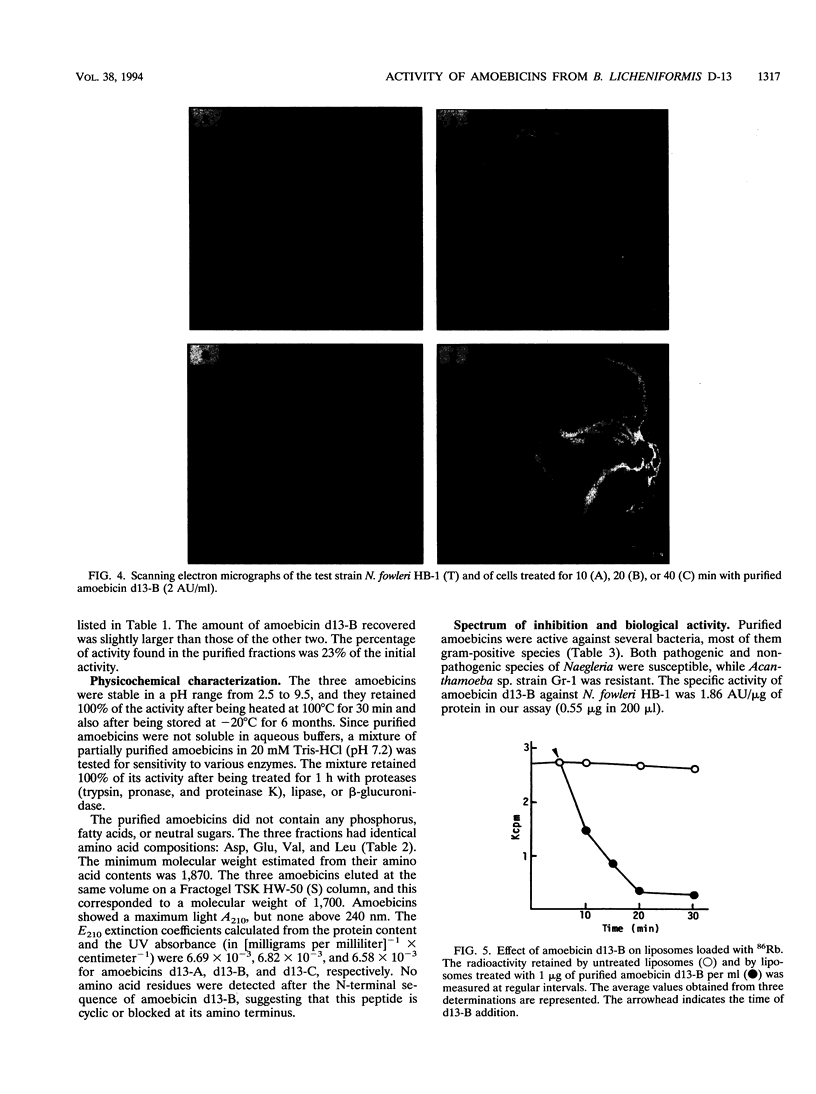
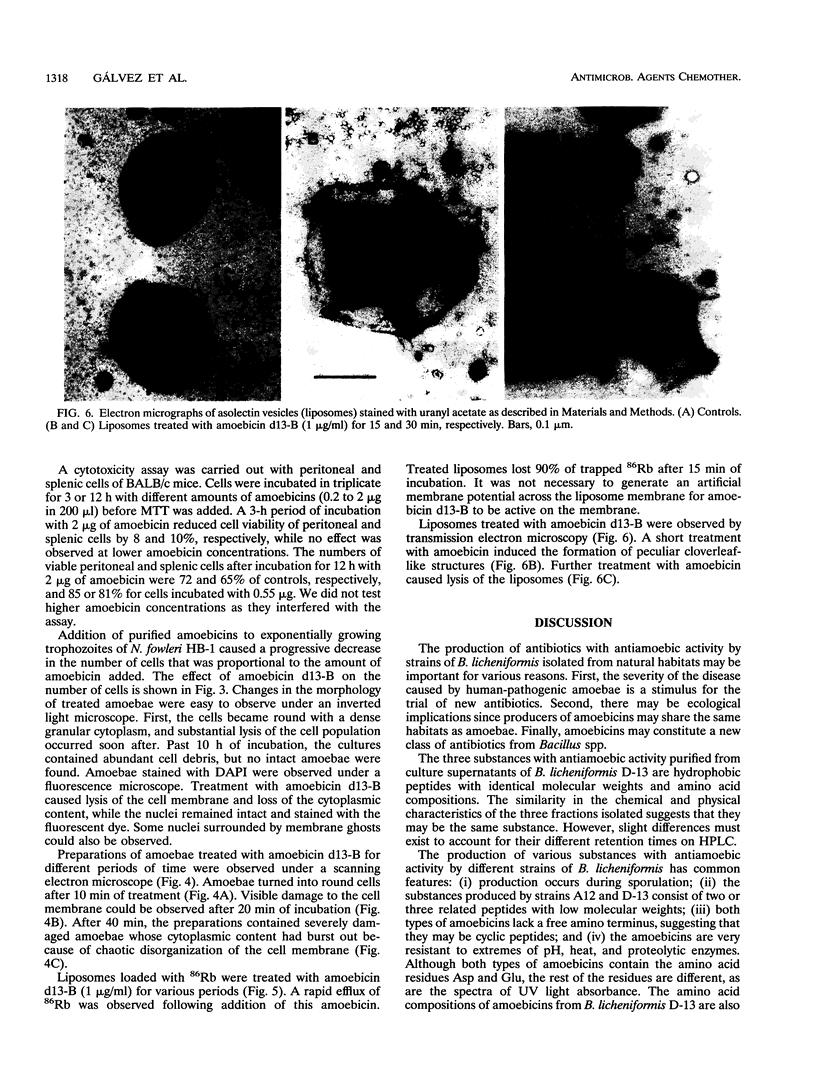

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CALLOW R. K., WORK T. S. Antibiotic peptides from Bacillus licheniformis; licheniformins A, B and C. Biochem J. 1952 Jul;51(4):558–568. doi: 10.1042/bj0510558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerva L. Amoebic meningoencephalitis: axenic culture of Naegleria. Science. 1969 Feb 7;163(3867):576–576. [PubMed] [Google Scholar]
- Cordovilla P., Valdivia E., Gonzalez-Segura A., Galvez A., Martinez-Bueno M., Maqueda M. Antagonistic action of the bacterium Bacillus licheniformis M-4 toward the amoeba Naegleria fowleri. J Eukaryot Microbiol. 1993 May-Jun;40(3):323–328. doi: 10.1111/j.1550-7408.1993.tb04923.x. [DOI] [PubMed] [Google Scholar]
- Cursons R. T., Brown T. J., Keys E. A., Gordon E. H., Leng R. H., Havill J. H., Hyne B. E. Primary amoebic meningo-encephalitis in an indoor heat-exchange swimming pool. N Z Med J. 1979 Oct 24;90(646):330–331. [PubMed] [Google Scholar]
- Gimenez-Gallego G., Thomas K. A. High-performance liquid chromatography of phenylthiocarbamyl-amino acids. Application to carboxyl-terminal sequencing of proteins. J Chromatogr. 1987 Nov 13;409:299–304. doi: 10.1016/s0021-9673(01)86806-0. [DOI] [PubMed] [Google Scholar]
- Gálvez A., Valdivia E., González-Segura A., Lebbadi M., Martínez-Bueno M., Maqueda M. Purification, characterization, and lytic activity against Naegleria fowleri of two amoebicins produced by Bacillus licheniformis A12. Appl Environ Microbiol. 1993 May;59(5):1480–1486. doi: 10.1128/aem.59.5.1480-1486.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haavik H. I. Studies on the formation of bacitracin by Bacillus licheniformis: effect of glucose. J Gen Microbiol. 1974 Apr;81(2):383–390. doi: 10.1099/00221287-81-2-383. [DOI] [PubMed] [Google Scholar]
- Ma P., Visvesvara G. S., Martinez A. J., Theodore F. H., Daggett P. M., Sawyer T. K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990 May-Jun;12(3):490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988 Mar;52(1):114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascaró C., Fluviá C., Osuna A., Guevara D. Virulent Naegleria sp. isolated from a river in Cadiz (Spain). J Parasitol. 1981 Aug;67(4):599–599. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nesemann G., Präve P., Sukatsch D., Vértesy L. Ein Polyen-Antibiotikum aus Bakterien. Naturwissenschaften. 1972 Feb;59(2):81–82. doi: 10.1007/BF00593477. [DOI] [PubMed] [Google Scholar]
- Pulverer G. Effects of cefodizime and cefotaxime on cellular and humoral immune responses. Infection. 1992;20 (Suppl 1):S41–S44. doi: 10.1007/BF01709950. [DOI] [PubMed] [Google Scholar]
- Scaglia M., Gatti S., Bernuzzi A. M., Cevini C., Chichino G., Rondanelli E. G. An in vitro comparative study on the effect of amphotericin B, econazole, and 5-fluorocytosine on Naegleria fowleri, Naegleria australiensis, and Naegleria australiensis s.sp. italica. Microbiologica. 1988 Oct;11(4):279–288. [PubMed] [Google Scholar]
- Scaglia M., Gatti S., Brustia R., Strosselli M., Bernuzzi A. M., Cevini C. Pathogenic and non-pathogenic Naegleria and Acanthamoeba spp.: a new autochthonous isolate from an Italian thermal area. Microbiologica. 1987 Apr;10(2):171–182. [PubMed] [Google Scholar]
- Schuster F. L., Mandel N. Phenothiazine compounds inhibit in vitro growth of pathogenic free-living amoebae. Antimicrob Agents Chemother. 1984 Jan;25(1):109–112. doi: 10.1128/aac.25.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidel J. S., Harmatz P., Visvesvara G. S., Cohen A., Edwards J., Turner J. Successful treatment of primary amebic meningoencephalitis. N Engl J Med. 1982 Feb 11;306(6):346–348. doi: 10.1056/NEJM198202113060607. [DOI] [PubMed] [Google Scholar]
- Sharples D., Barbe J., Galy A. M., Galy J. P. New antiamebic acridines. II. Synthesis and DNA binding of a series of 9-acridanones and 9-iminoacridines. Chemotherapy. 1987;33(5):347–354. doi: 10.1159/000238520. [DOI] [PubMed] [Google Scholar]
- Smego R. A., Jr, Durack D. T. In vitro susceptibility testing of Naegleria fowleri to ketoconazole, BAYn7133, and allopurinol riboside. J Parasitol. 1984 Apr;70(2):317–318. [PubMed] [Google Scholar]
- Tyndall R. L., Ironside K. S., Metler P. L., Tan E. L., Hazen T. C., Fliermans C. B. Effect of thermal additions on the density and distribution of thermophilic amoebae and pathogenic Naegleria fowleri in a newly created cooling lake. Appl Environ Microbiol. 1989 Mar;55(3):722–732. doi: 10.1128/aem.55.3.722-732.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings F. M., Amuso P. T., Chang S. L., Lewis A. L. Isolation and identification of pathogenic Naegleria from Florida lakes. Appl Environ Microbiol. 1977 Dec;34(6):661–667. doi: 10.1128/aem.34.6.661-667.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]