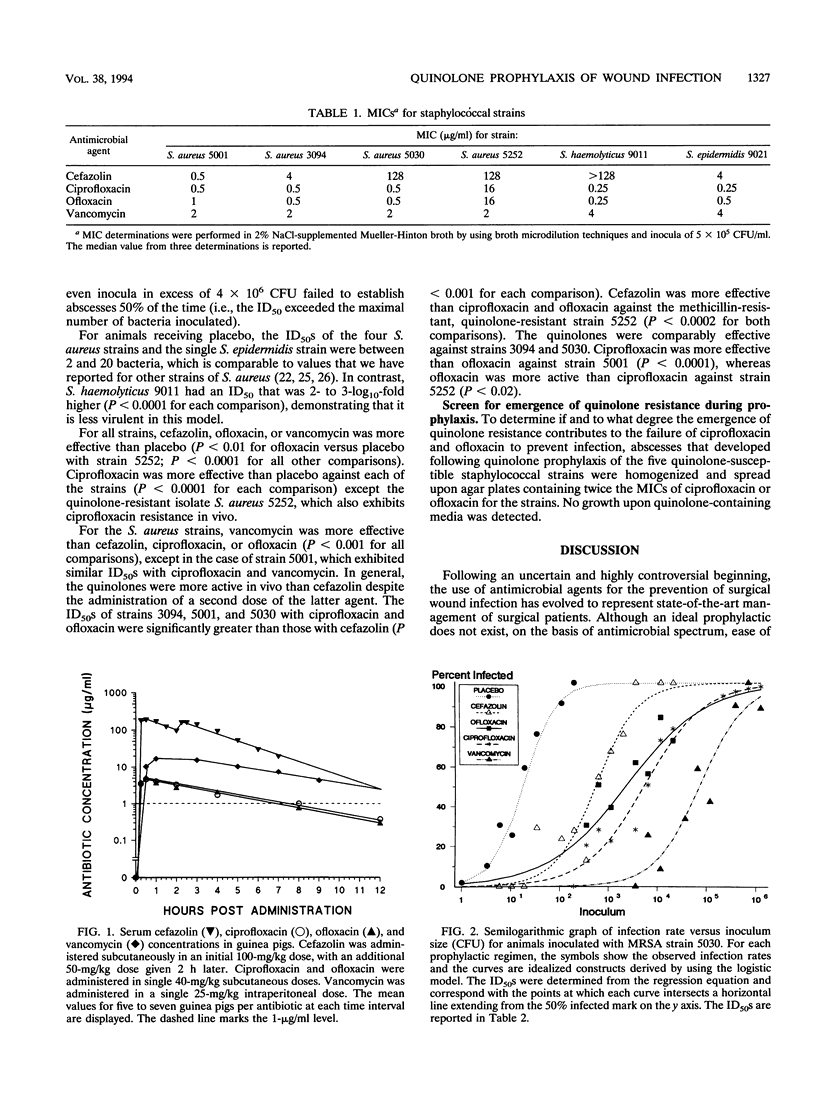
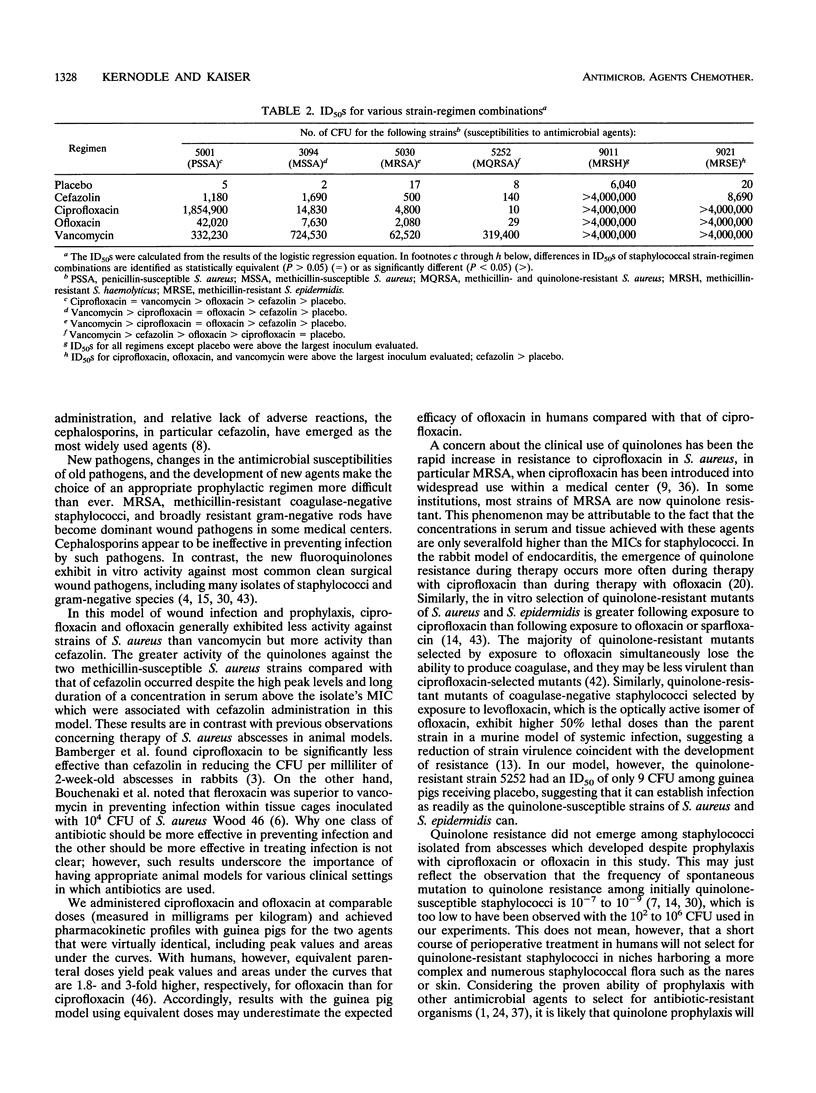
Abstract
Recent shifts in the species and antibiotic resistance patterns of bacteria causing nosocomial infections present new challenges for providing effective prophylaxis in surgery. Traditional regimens lack activity against methicillin-resistant staphylococci and many gram-negative species causing nosocomial infections. The new fluoroquinolones exhibit in vitro activity against many emerging surgical wound pathogens. To determine the potential of this class of antimicrobial agents for use in surgery, we compared the prophylactic efficacies of ciprofloxacin and ofloxacin with those of cefazolin and vancomycin in a guinea pig model of abscess formation. Four Staphylococcus aureus strains, one Staphylococcus epidermidis strain, and one Staphylococcus haemolyticus strain were evaluated. Vancomycin was the most effective prophylactic agent, exhibiting in vivo activity against all strains which was superior or equivalent to those of all other agents tested. Cefazolin was the least effective agent and surpassed the two quinolones in prophylactic efficacy against only one organism, a quinolone- and methicillin-resistant strain of S. aureus. The two quinolones provided excellent protection against infection with all but the quinolone-resistant isolate. The in vivo emergence of quinolone resistance among quinolone-susceptible isolates was not detected. The methicillin-resistant, quinolone-susceptible S. epidermidis and S. haemolyticus isolates were extremely susceptible to prophylaxis, exhibiting 50% infective doses above 4 x 10(6) CFU for seven of the eight antibiotic-strain combinations. We conclude that ciprofloxacin and ofloxacin may be effective antistaphylococcal agents in surgery. The role of these agents remains to be defined, and the definition should include consideration of an adverse effect upon antibiotic resistance patterns of organisms causing nosocomial infections.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archer G. L., Dietrick D. R., Johnston J. L. Molecular epidemiology of transmissible gentamicin resistance among coagulase-negative staphylococci in a cardiac surgery unit. J Infect Dis. 1985 Feb;151(2):243–251. doi: 10.1093/infdis/151.2.243. [DOI] [PubMed] [Google Scholar]
- Auten G. M., Preheim L. C., Sookpranee M., Bittner M. J., Sookpranee T., Vibhagool A. High-pressure liquid chromatography and microbiological assay of serum ofloxacin levels in adults receiving intravenous and oral therapy for skin infections. Antimicrob Agents Chemother. 1991 Dec;35(12):2558–2561. doi: 10.1128/aac.35.12.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamberger D. M., Fields M. T., Herndon B. L. Efficacies of various antimicrobial agents in treatment of Staphylococcus aureus abscesses and correlation with in vitro tests of antimicrobial activity and neutrophil killing. Antimicrob Agents Chemother. 1991 Nov;35(11):2335–2339. doi: 10.1128/aac.35.11.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry A. L., Fuchs P. C. Anti-staphylococcal activity of temafloxacin, ciprofloxacin, ofloxacin and enoxacin. J Antimicrob Chemother. 1991 Nov;28(5):695–699. doi: 10.1093/jac/28.5.695. [DOI] [PubMed] [Google Scholar]
- Blomstedt G. C., Kyttä J. Results of a randomized trial of vancomycin prophylaxis in craniotomy. J Neurosurg. 1988 Aug;69(2):216–220. doi: 10.3171/jns.1988.69.2.0216. [DOI] [PubMed] [Google Scholar]
- Bouchenaki N., Vaudaux P. E., Huggler E., Waldvogel F. A., Lew D. P. Successful single-dose prophylaxis of Staphylococcus aureus foreign body infections in guinea pigs by fleroxacin. Antimicrob Agents Chemother. 1990 Jan;34(1):21–24. doi: 10.1128/aac.34.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullmann W., Stieglitz M., Baars B., Opferkuch W. Comparative evaluation of recently developed quinolone compounds--with a note on the frequency of resistant mutants. Chemotherapy. 1985;31(1):19–28. doi: 10.1159/000238309. [DOI] [PubMed] [Google Scholar]
- Currier J. S., Campbell H., Platt R., Kaiser A. B. Perioperative antimicrobial prophylaxis in middle Tennessee, 1989-1990. Rev Infect Dis. 1991 Sep-Oct;13 (Suppl 10):S874–S878. [PubMed] [Google Scholar]
- Daum T. E., Schaberg D. R., Terpenning M. S., Sottile W. S., Kauffman C. A. Increasing resistance of Staphylococcus aureus to ciprofloxacin. Antimicrob Agents Chemother. 1990 Sep;34(9):1862–1863. doi: 10.1128/aac.34.9.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhawan V. K., Maier M. K., Nayyar M., Chuah S. K., Thadepalli H. In vitro activity of cefmenoxime, cefotaxime, latamoxef, cefazolin, nafcillin and vancomycin against 53 endocarditis and bacteremic strains of Staphylococcus aureus. Chemotherapy. 1984;30(5):328–330. doi: 10.1159/000238288. [DOI] [PubMed] [Google Scholar]
- Farrar W. E., Jr, Kent T. H. Enteritis and coliform bacteremia in guinea pigs given penicillin. Am J Pathol. 1965 Oct;47(4):629–642. [PMC free article] [PubMed] [Google Scholar]
- Flor S. Pharmacokinetics of ofloxacin. An overview. Am J Med. 1989 Dec 29;87(6C):24S–30S. [PubMed] [Google Scholar]
- Foleno B. D., Lafredo S. C., Fu K. P. In vitro activity of levofloxacin, ofloxacin and other quinolones against coagulase-negative staphylococci. Chemotherapy. 1993 Mar-Apr;39(2):120–123. doi: 10.1159/000239112. [DOI] [PubMed] [Google Scholar]
- Forstall G. J., Knapp C. C., Washington J. A. Activity of new quinolones against ciprofloxacin-resistant staphylococci. Antimicrob Agents Chemother. 1991 Aug;35(8):1679–1681. doi: 10.1128/aac.35.8.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs P. C. In vitro antimicrobial activity and susceptibility testing of ofloxacin. Current status. Am J Med. 1989 Dec 29;87(6C):10S–13S. [PubMed] [Google Scholar]
- Geraghty J., Feely M. Antibiotic prophylaxis in neurosurgery. A randomized controlled trial. J Neurosurg. 1984 Apr;60(4):724–726. doi: 10.3171/jns.1984.60.4.0724. [DOI] [PubMed] [Google Scholar]
- Hessen M. T., Pitsakis P. G., Kaye D. Oral temafloxacin versus vancomycin for therapy of experimental endocarditis caused by methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1990 Jun;34(6):1143–1145. doi: 10.1128/aac.34.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen L. J., Aagaard M. T., Schifter S. Prophylactic vancomycin versus placebo in arterial prosthetic reconstructions. Thorac Cardiovasc Surg. 1985 Oct;33(5):300–303. doi: 10.1055/s-2007-1014145. [DOI] [PubMed] [Google Scholar]
- Joyce F. S., Szczepanski K. P. A double-blind comparative study of prophylactic antibiotic therapy in open heart surgery: penicillin G versus vancomycin. Thorac Cardiovasc Surg. 1986 Apr;34(2):100–103. doi: 10.1055/s-2007-1020387. [DOI] [PubMed] [Google Scholar]
- Kaatz G. W., Seo S. M., Barriere S. L., Albrecht L. M., Rybak M. J. Efficacy of ofloxacin in experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990 Feb;34(2):257–260. doi: 10.1128/aac.34.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser A. B., Kernodle D. S., Parker R. A. Low-inoculum model of surgical wound infection. J Infect Dis. 1992 Aug;166(2):393–399. doi: 10.1093/infdis/166.2.393. [DOI] [PubMed] [Google Scholar]
- Kaiser A. B. Overview of cephalosporin prophylaxis. Am J Surg. 1988 May 31;155(5A):52–55. doi: 10.1016/s0002-9610(88)80213-7. [DOI] [PubMed] [Google Scholar]
- Kernodle D. S., Barg N. L., Kaiser A. B. Low-level colonization of hospitalized patients with methicillin-resistant coagulase-negative staphylococci and emergence of the organisms during surgical antimicrobial prophylaxis. Antimicrob Agents Chemother. 1988 Feb;32(2):202–208. doi: 10.1128/aac.32.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kernodle D. S., Kaiser A. B. Comparative prophylactic efficacy of cefazolin and vancomycin in a guinea pig model of Staphylococcus aureus wound infection. J Infect Dis. 1993 Jul;168(1):152–157. doi: 10.1093/infdis/168.1.152. [DOI] [PubMed] [Google Scholar]
- Kernodle D. S., Kaiser A. B. Efficacy of prophylaxis with beta-lactams and beta-lactam-beta-lactamase inhibitor combinations against wound infection by methicillin-resistant and borderline-susceptible Staphylococcus aureus in a guinea pig model. Antimicrob Agents Chemother. 1993 Apr;37(4):702–707. doi: 10.1128/aac.37.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshbaum A., Arret B. Outline of details for official microbiological assays of antibiotics. J Pharm Sci. 1967 Apr;56(4):511–515. doi: 10.1002/jps.2600560418. [DOI] [PubMed] [Google Scholar]
- Lamp K. C., Bailey E. M., Rybak M. J. Ofloxacin clinical pharmacokinetics. Clin Pharmacokinet. 1992 Jan;22(1):32–46. doi: 10.2165/00003088-199222010-00004. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Bohn M. J., Stolz S. M., Kroncke G. M., Acher C. W., Myerowitz P. D. Comparative study of cefazolin, cefamandole, and vancomycin for surgical prophylaxis in cardiac and vascular operations. A double-blind randomized trial. J Thorac Cardiovasc Surg. 1992 Nov;104(5):1423–1434. [PubMed] [Google Scholar]
- Maple P. A., Hamilton-Miller J. M., Brumfitt W. Differing activities of quinolones against ciprofloxacin-susceptible and ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1991 Feb;35(2):345–350. doi: 10.1128/aac.35.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newfield P., Roizen M. F. Hazards of rapid administration of vancomycin. Ann Intern Med. 1979 Oct;91(4):581–581. doi: 10.7326/0003-4819-91-4-581. [DOI] [PubMed] [Google Scholar]
- Panlilio A. L., Culver D. H., Gaynes R. P., Banerjee S., Henderson T. S., Tolson J. S., Martone W. J. Methicillin-resistant Staphylococcus aureus in U.S. hospitals, 1975-1991. Infect Control Hosp Epidemiol. 1992 Oct;13(10):582–586. doi: 10.1086/646432. [DOI] [PubMed] [Google Scholar]
- Polk R. E., Healy D. P., Schwartz L. B., Rock D. T., Garson M. L., Roller K. Vancomycin and the red-man syndrome: pharmacodynamics of histamine release. J Infect Dis. 1988 Mar;157(3):502–507. doi: 10.1093/infdis/157.3.502. [DOI] [PubMed] [Google Scholar]
- Raviglione M. C., Boyle J. F., Mariuz P., Pablos-Mendez A., Cortes H., Merlo A. Ciprofloxacin-resistant methicillin-resistant Staphylococcus aureus in an acute-care hospital. Antimicrob Agents Chemother. 1990 Nov;34(11):2050–2054. doi: 10.1128/aac.34.11.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts N. J., Jr, Douglas R. G., Jr Gentamicin use and Pseudomonas and Serratia resistance: effect of a surgical prophylaxis regimen. Antimicrob Agents Chemother. 1978 Feb;13(2):214–220. doi: 10.1128/aac.13.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaberg D. R., Culver D. H., Gaynes R. P. Major trends in the microbial etiology of nosocomial infection. Am J Med. 1991 Sep 16;91(3B):72S–75S. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- Schwenzer K. S., Wang C. H., Anhalt J. P. Automated fluorescence polarization immunoassay for monitoring vancomycin. Ther Drug Monit. 1983;5(3):341–345. doi: 10.1097/00007691-198309000-00017. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Townsend T. R., Rosner B., Kass E. H. Use of antimicrobial drugs in general hospitals: patterns of prophylaxis. N Engl J Med. 1979 Aug 16;301(7):351–355. doi: 10.1056/NEJM197908163010703. [DOI] [PubMed] [Google Scholar]
- Shapiro M., Wald U., Simchen E., Pomeranz S., Zagzag D., Michowiz S. D., Samuel-Cahn E., Wax Y., Shuval R., Kahane Y. Randomized clinical trial of intra-operative antimicrobial prophylaxis of infection after neurosurgical procedures. J Hosp Infect. 1986 Nov;8(3):283–295. doi: 10.1016/0195-6701(86)90125-8. [DOI] [PubMed] [Google Scholar]
- Smith J. T. Mutation rates to 4-quinolone resistance. Arzneimittelforschung. 1990 Jan;40(1):65–68. [PubMed] [Google Scholar]
- Smith K. R., Cobbs C. G. In vitro activity of sparfloxacin and three other fluoroquinolones against methicillin-resistant Staphylococcus aureus and Staphylococcus epidermidis. Eur J Clin Microbiol Infect Dis. 1992 Jan;11(1):55–58. doi: 10.1007/BF01971273. [DOI] [PubMed] [Google Scholar]
- Vander Salm T. J., Okike O. N., Pasque M. K., Pezzella A. T., Lew R., Traina V., Mathieu R. Reduction of sternal infection by application of topical vancomycin. J Thorac Cardiovasc Surg. 1989 Oct;98(4):618–622. [PubMed] [Google Scholar]
- Wenzel R. P. Preoperative antibiotic prophylaxis. N Engl J Med. 1992 Jan 30;326(5):337–339. doi: 10.1056/NEJM199201303260509. [DOI] [PubMed] [Google Scholar]
- Wolfson J. S., Hooper D. C. Comparative pharmacokinetics of ofloxacin and ciprofloxacin. Am J Med. 1989 Dec 29;87(6C):31S–36S. [PubMed] [Google Scholar]



