Abstract
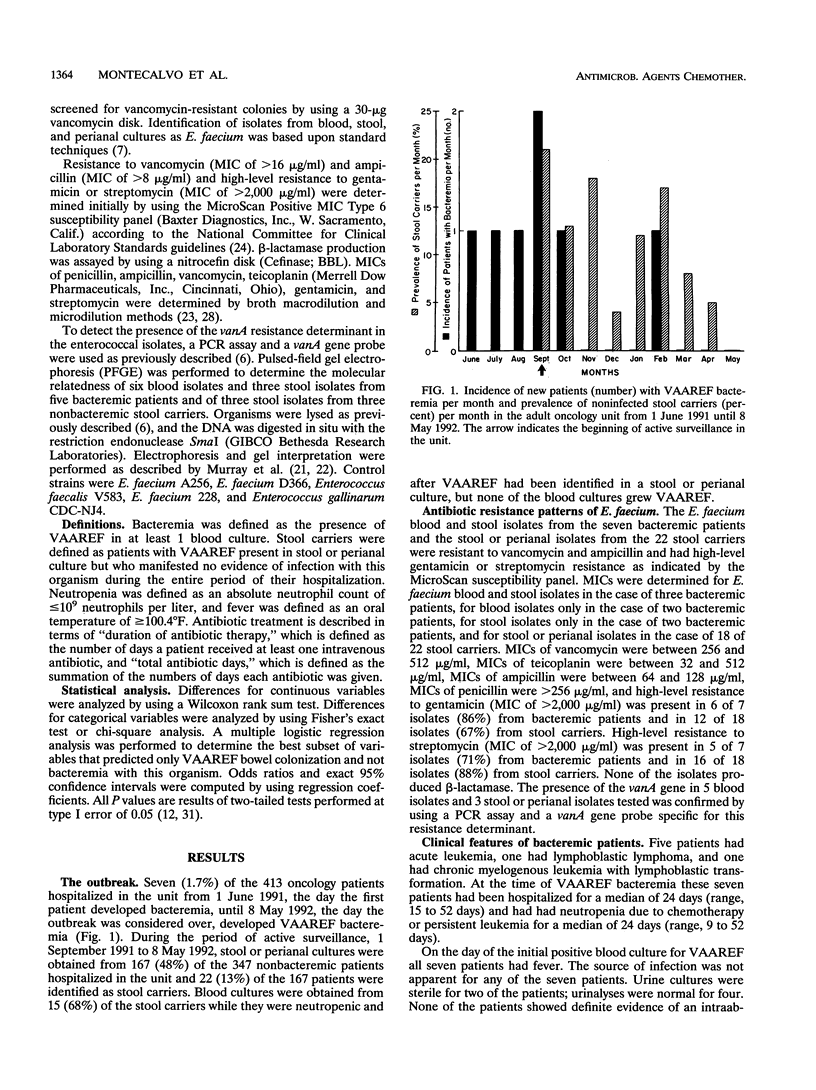
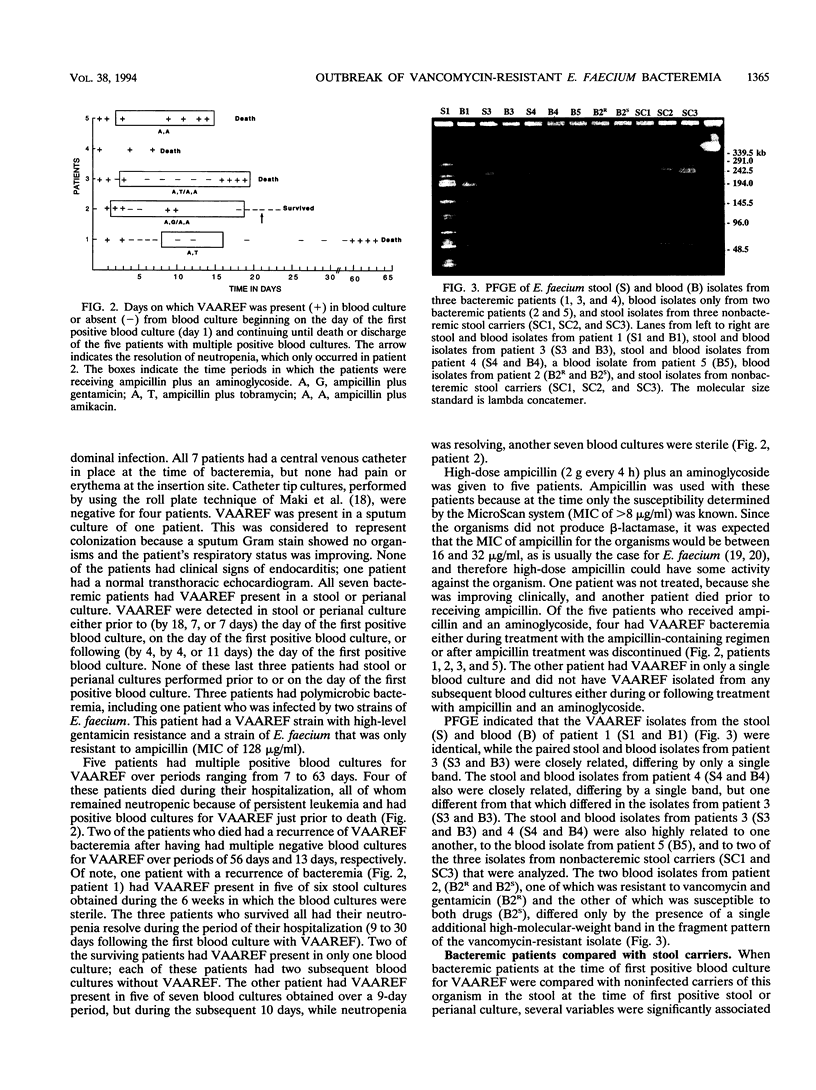
An outbreak of bacteremia caused by Enterococcus faecium with high-level resistance to vancomycin (MIC of > or = 256 micrograms/ml), ampicillin (MIC of > or = 64 micrograms/ml), and gentamicin or streptomycin (MIC of > or = 2,000 micrograms/ml) occurred in an adult oncology unit from June 1991 to May 1992. Active surveillance for the presence of this organism in stool or perianal cultures was begun in September 1991. Between June 1991 and May 1992, seven patients with bacteremia and 22 noninfected carriers of the organism in stool were identified. The vanA gene, tested for by PCR and gene probe, was present in all isolates evaluated. All bacteremic patients also had resistant E. faecium present in a stool or perianal culture; the stool isolates tested were closely related to the respective blood isolates as determined by pulsed-field gel electrophoresis. Antibiotic regimens using high-dose ampicillin and an aminoglycoside were ineffective with four patients. Five patients (71%) had multiple positive blood cultures; four of these patients died. Following a multiple logistic regression analysis, it was found that bacteremic patients received a significantly greater number of total antibiotic days compared with noninfected stool carriers (P = 0.019). The emergence of E. faecium with high-level resistance to vancomycin, ampicillin, and aminoglycosides underscores the importance of performing susceptibility testing on all clinically significant isolates. In the neutropenic adult oncology patient, bacteremia with this organism is of probable gastrointestinal origin, is often persistent, and is refractory to treatment with ampicillin in combination with an aminoglycoside. Prolonged use of antibiotics may predispose patients with gastrointestinal colonization to develop bacteremia.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alavi J. B., Root R. K., Djerassi I., Evans A. E., Gluckman S. J., MacGregor R. R., Guerry D., Schreiber A. D., Shaw J. M., Koch P. A randomized clinical trial of granulocyte transfusions for infection in acute leukemia. N Engl J Med. 1977 Mar 31;296(13):706–711. doi: 10.1056/NEJM197703312961302. [DOI] [PubMed] [Google Scholar]
- Awada A., van der Auwera P., Meunier F., Daneau D., Klastersky J. Streptococcal and enterococcal bacteremia in patients with cancer. Clin Infect Dis. 1992 Jul;15(1):33–48. doi: 10.1093/clinids/15.1.33. [DOI] [PubMed] [Google Scholar]
- Bush L. M., Calmon J., Cherney C. L., Wendeler M., Pitsakis P., Poupard J., Levison M. E., Johnson C. C. High-level penicillin resistance among isolates of enterococci. Implications for treatment of enterococcal infections. Ann Intern Med. 1989 Apr 1;110(7):515–520. doi: 10.7326/0003-4819-110-7-515. [DOI] [PubMed] [Google Scholar]
- Calderwood S. A., Wennersten C., Moellering R. C., Jr, Kunz L. J., Krogstad D. J. Resistance to six aminoglycosidic aminocyclitol antibiotics among enterococci: prevalence, evolution, and relationship to synergism with penicillin. Antimicrob Agents Chemother. 1977 Sep;12(3):401–405. doi: 10.1128/aac.12.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark N. C., Cooksey R. C., Hill B. C., Swenson J. M., Tenover F. C. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993 Nov;37(11):2311–2317. doi: 10.1128/aac.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson M. L., Eliopoulos G. M., Wennersten C. B., Ruoff K. L., De Girolami P. C., Ferraro M. J., Moellering R. C., Jr Increasing resistance to beta-lactam antibiotics among clinical isolates of Enterococcus faecium: a 22-year review at one institution. Antimicrob Agents Chemother. 1991 Nov;35(11):2180–2184. doi: 10.1128/aac.35.11.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerger S., Perlman D. C., Altarac D., McAuliffe V. Concomitant high-level vancomycin and penicillin resistance in clinical isolates of enterococci. Clin Infect Dis. 1992 Mar;14(3):655–661. doi: 10.1093/clinids/14.3.655. [DOI] [PubMed] [Google Scholar]
- Handwerger S., Raucher B., Altarac D., Monka J., Marchione S., Singh K. V., Murray B. E., Wolff J., Walters B. Nosocomial outbreak due to Enterococcus faecium highly resistant to vancomycin, penicillin, and gentamicin. Clin Infect Dis. 1993 Jun;16(6):750–755. doi: 10.1093/clind/16.6.750. [DOI] [PubMed] [Google Scholar]
- Horodniceanu T., Bougueleret L., El-Solh N., Bieth G., Delbos F. High-level, plasmid-borne resistance to gentamicin in Streptococcus faecalis subsp. zymogenes. Antimicrob Agents Chemother. 1979 Nov;16(5):686–689. doi: 10.1128/aac.16.5.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanfil L. V., Murphy M., Josephson A., Gaynes R., Mandel L., Hill B. C., Swenson J. M. A cluster of vancomycin-resistant Enterococcus faecium in an intensive care unit. Infect Control Hosp Epidemiol. 1992 Apr;13(4):195–200. doi: 10.1086/646509. [DOI] [PubMed] [Google Scholar]
- Karp J. E., Dick J. D., Angelopulos C., Charache P., Green L., Burke P. J., Saral R. Empiric use of vancomycin during prolonged treatment-induced granulocytopenia. Randomized, double-blind, placebo-controlled clinical trial in patients with acute leukemia. Am J Med. 1986 Aug;81(2):237–242. doi: 10.1016/0002-9343(86)90257-3. [DOI] [PubMed] [Google Scholar]
- Leclercq R., Derlot E., Duval J., Courvalin P. Plasmid-mediated resistance to vancomycin and teicoplanin in Enterococcus faecium. N Engl J Med. 1988 Jul 21;319(3):157–161. doi: 10.1056/NEJM198807213190307. [DOI] [PubMed] [Google Scholar]
- Livornese L. L., Jr, Dias S., Samel C., Romanowski B., Taylor S., May P., Pitsakis P., Woods G., Kaye D., Levison M. E. Hospital-acquired infection with vancomycin-resistant Enterococcus faecium transmitted by electronic thermometers. Ann Intern Med. 1992 Jul 15;117(2):112–116. doi: 10.7326/0003-4819-117-2-112. [DOI] [PubMed] [Google Scholar]
- Lynch P., Jackson M. M., Cummings M. J., Stamm W. E. Rethinking the role of isolation practices in the prevention of nosocomial infections. Ann Intern Med. 1987 Aug;107(2):243–246. doi: 10.7326/0003-4819-107-2-243. [DOI] [PubMed] [Google Scholar]
- Maki D. G., Weise C. E., Sarafin H. W. A semiquantitative culture method for identifying intravenous-catheter-related infection. N Engl J Med. 1977 Jun 9;296(23):1305–1309. doi: 10.1056/NEJM197706092962301. [DOI] [PubMed] [Google Scholar]
- Moellering R. C., Jr, Korzeniowski O. M., Sande M. A., Wennersten C. B. Species-specific resistance to antimocrobial synergism in Streptococcus faecium and Streptococcus faecalis. J Infect Dis. 1979 Aug;140(2):203–208. doi: 10.1093/infdis/140.2.203. [DOI] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Heath J. D., Sharma B. R., Weinstock G. M. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J Clin Microbiol. 1990 Sep;28(9):2059–2063. doi: 10.1128/jcm.28.9.2059-2063.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. E., Singh K. V., Markowitz S. M., Lopardo H. A., Patterson J. E., Zervos M. J., Rubeglio E., Eliopoulos G. M., Rice L. B., Goldstein F. W. Evidence for clonal spread of a single strain of beta-lactamase-producing Enterococcus (Streptococcus) faecalis to six hospitals in five states. J Infect Dis. 1991 Apr;163(4):780–785. doi: 10.1093/infdis/163.4.780. [DOI] [PubMed] [Google Scholar]
- Murray B. E. The life and times of the Enterococcus. Clin Microbiol Rev. 1990 Jan;3(1):46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachamkin I., Axelrod P., Talbot G. H., Fischer S. H., Wennersten C. B., Moellering R. C., Jr, MacGregor R. R. Multiply high-level-aminoglycoside-resistant enterococci isolated from patients in a university hospital. J Clin Microbiol. 1988 Jul;26(7):1287–1291. doi: 10.1128/jcm.26.7.1287-1291.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzo P. A., Hathorn J. W., Hiemenz J., Browne M., Commers J., Cotton D., Gress J., Longo D., Marshall D., McKnight J. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. N Engl J Med. 1986 Aug 28;315(9):552–558. doi: 10.1056/NEJM198608283150905. [DOI] [PubMed] [Google Scholar]
- Rubin L. G., Tucci V., Cercenado E., Eliopoulos G., Isenberg H. D. Vancomycin-resistant Enterococcus faecium in hospitalized children. Infect Control Hosp Epidemiol. 1992 Dec;13(12):700–705. doi: 10.1086/648342. [DOI] [PubMed] [Google Scholar]
- Rubin M., Hathorn J. W., Marshall D., Gress J., Steinberg S. M., Pizzo P. A. Gram-positive infections and the use of vancomycin in 550 episodes of fever and neutropenia. Ann Intern Med. 1988 Jan;108(1):30–35. doi: 10.7326/0003-4819-108-1-30. [DOI] [PubMed] [Google Scholar]



