Abstract
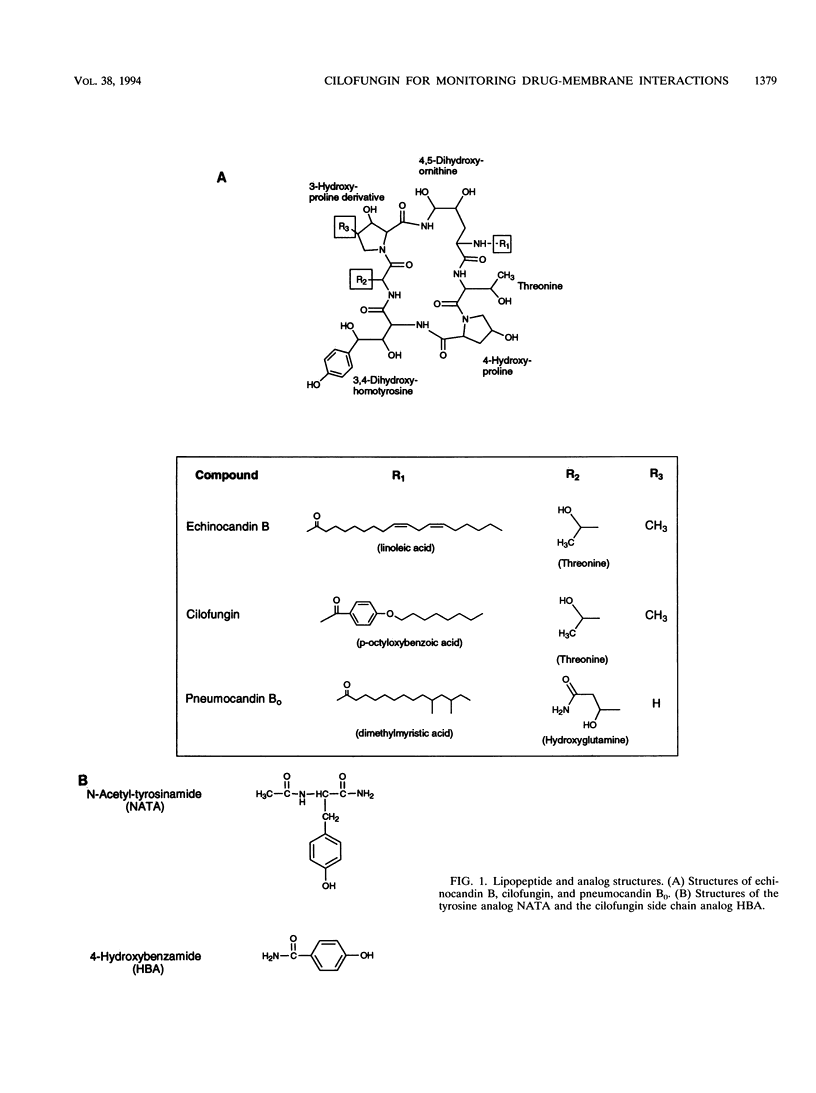
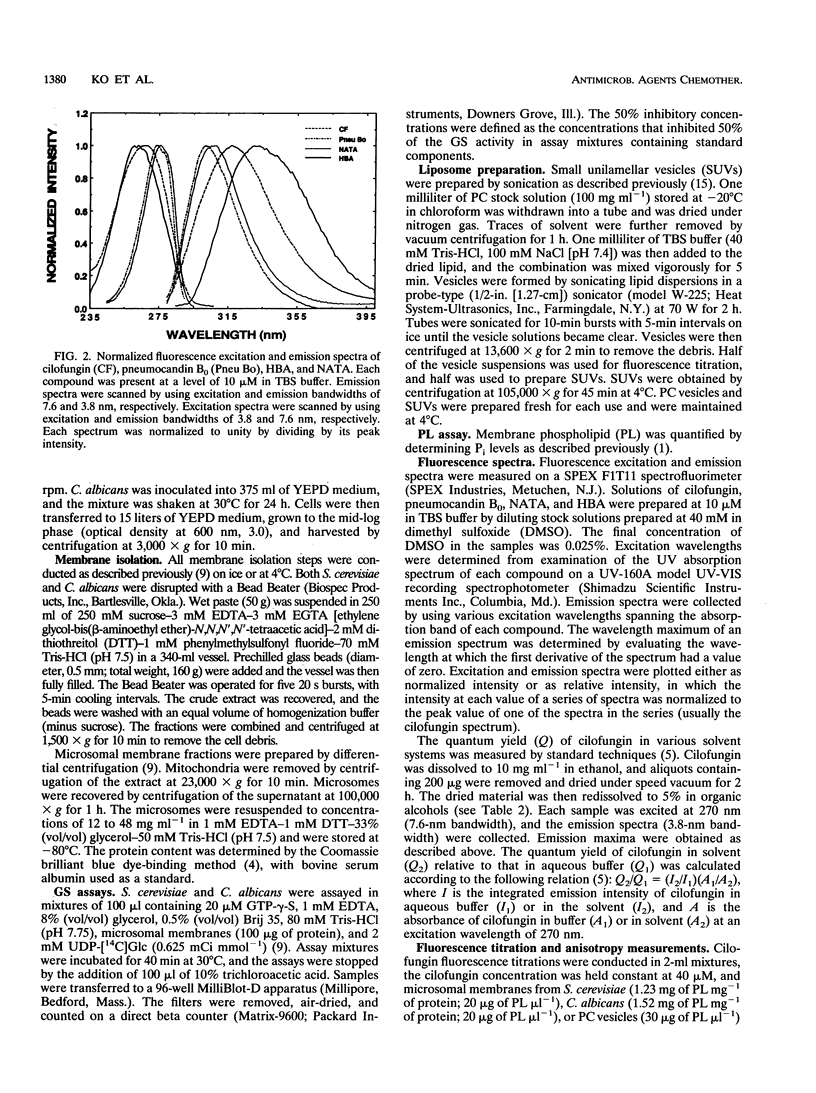
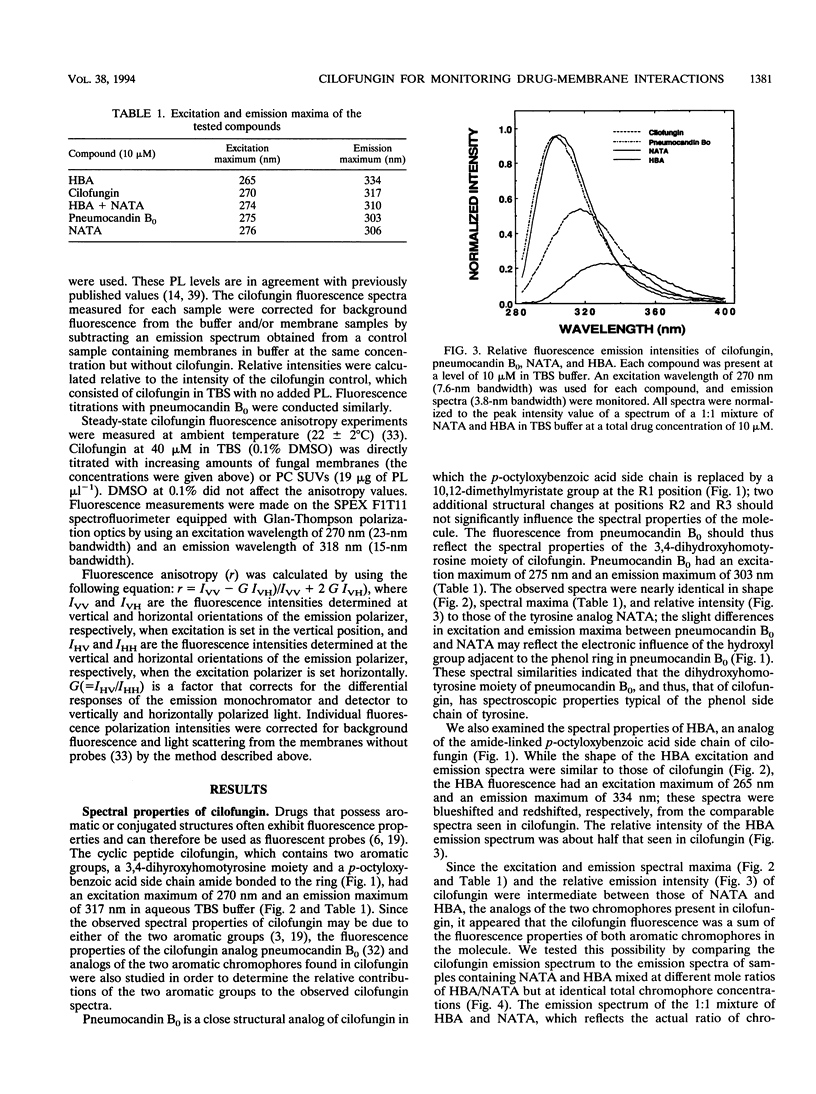
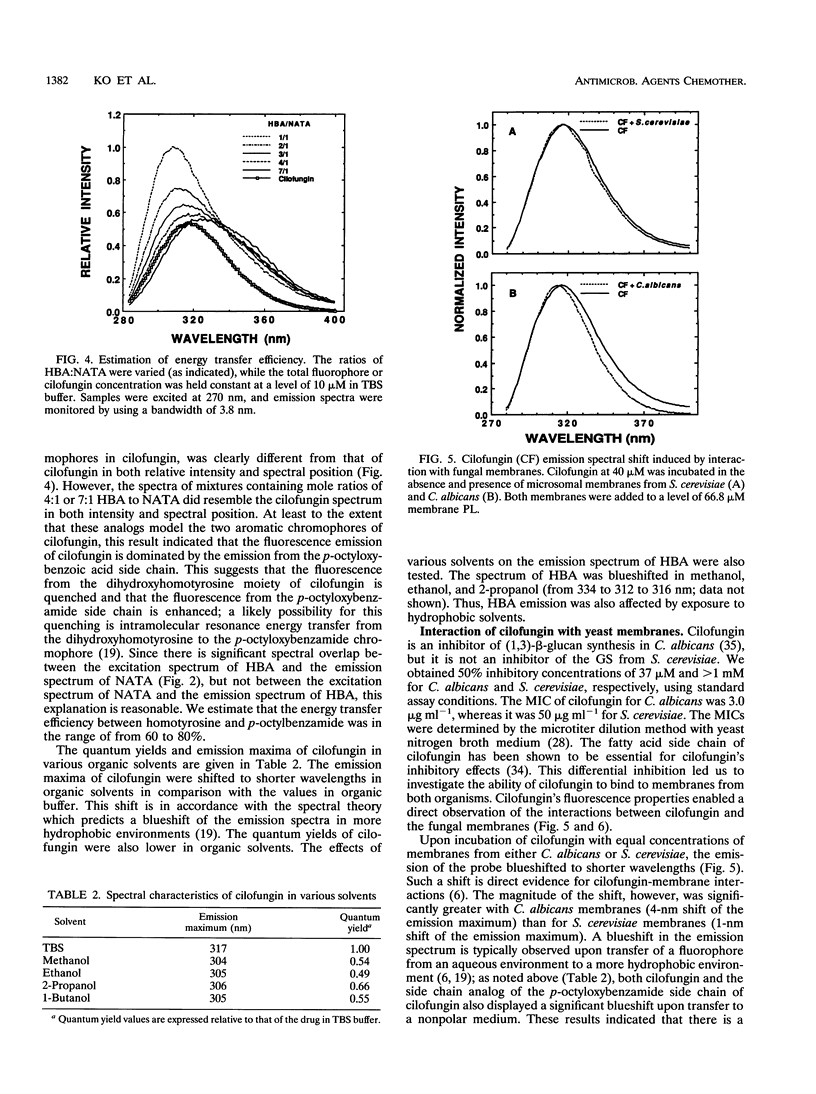
Cilofungin is an antifungal cyclopeptide which inhibits cell wall (1,3)-beta-glucan biosynthesis in fungal organisms, and its action against Candida albicans (1,3)-beta-glucan synthase has been widely studied. Since glucan synthase inactivation is thought to partially result from perturbations of the membrane lipid environment, the interaction of cilofungin with fungal membranes and phosphatidylcholine membrane vesicles was studied. Cilofungin, which contains two independent aromatic groups, has an excitation maximum of 270 nm and an emission maximum of 317 nm in aqueous solution. Comparison of the fluorescence properties of cilofungin with those of the analogs pneumocandin B0, N-acetyl-tyrosinamide, and 4-hydroxybenzamide indicated that the emission of cilofungin largely derived from the p-octyloxybenzamide side chain. Microsomal membranes from Saccharomyces cerevisiae, C. albicans, and phosphatidylcholine membrane vesicles induced a blue shift in the cilofungin emission spectrum and increased the cilofungin steady-state emission anisotropy, providing direct evidence for a cilofungin-membrane interaction. Cilofungin interacted more strongly with membranes of C. albicans than with those of S. cerevisiae, correlating with previous findings that C. albicans is far more susceptible than S. cerevisiae to the action of cilofungin. These findings support the hypothesis that drug-induced inhibition of the (1,3)-beta-glucan synthesis results from the perturbation of the membrane environment and the interaction with the glucan synthase complex combined. The study demonstrated ways in which the fluorescence properties of drugs can be used to directly evaluate drug-membrane interactions and structure-activity relationships.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Römmele G., Gruner J., Wehrli W. Papulacandin B: an inhibitor of glucan synthesis in yeast spheroplasts. Eur J Biochem. 1979 Jul;97(2):345–351. doi: 10.1111/j.1432-1033.1979.tb13120.x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burke T. G., Mishra A. K., Wani M. C., Wall M. E. Lipid bilayer partitioning and stability of camptothecin drugs. Biochemistry. 1993 May 25;32(20):5352–5364. doi: 10.1021/bi00071a010. [DOI] [PubMed] [Google Scholar]
- Burke T. G., Tritton T. R. Location and dynamics of anthracyclines bound to unilamellar phosphatidylcholine vesicles. Biochemistry. 1985 Oct 8;24(21):5972–5980. doi: 10.1021/bi00342a043. [DOI] [PubMed] [Google Scholar]
- Debono M., Abbott B. J., Turner J. R., Howard L. C., Gordee R. S., Hunt A. S., Barnhart M., Molloy R. M., Willard K. E., Fukuda D. Synthesis and evaluation of LY121019, a member of a series of semisynthetic analogues of the antifungal lipopeptide echinocandin B. Ann N Y Acad Sci. 1988;544:152–167. doi: 10.1111/j.1749-6632.1988.tb40398.x. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Ellis L. F., Thakkar A. L., Howard L. C. In vitro and in vivo anti-Candida activity and toxicology of LY121019. J Antibiot (Tokyo) 1984 Sep;37(9):1054–1065. doi: 10.7164/antibiotics.37.1054. [DOI] [PubMed] [Google Scholar]
- Gordee R. S., Zeckner D. J., Howard L. C., Alborn W. E., Jr, Debono M. Anti-Candida activity and toxicology of LY121019, a novel semisynthetic polypeptide antifungal antibiotic. Ann N Y Acad Sci. 1988;544:294–309. doi: 10.1111/j.1749-6632.1988.tb40415.x. [DOI] [PubMed] [Google Scholar]
- Henry-Toulmé N., Seman M., Bolard J. Interaction of amphotericin B and its N-fructosyl derivative with murine thymocytes: a comparative study using fluorescent membrane probes. Biochim Biophys Acta. 1989 Jul 10;982(2):245–252. doi: 10.1016/0005-2736(89)90061-8. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition and permeability to the triazole antifungal antibiotic ICI 153066 of serum-grown mycelial cultures of Candida albicans. J Gen Microbiol. 1989 Jul;135(7):1949–1955. doi: 10.1099/00221287-135-7-1949. [DOI] [PubMed] [Google Scholar]
- Huang C., Thompson T. E. Preparation of homogeneous, single-walled phosphatidylcholine vesicles. Methods Enzymol. 1974;32:485–489. doi: 10.1016/0076-6879(74)32048-4. [DOI] [PubMed] [Google Scholar]
- Kang M. S., Szaniszlo P. J., Notario V., Cabib E. The effect of papulacandin B on (1----3)-beta-D-glucan synthetases. A possible relationship between inhibition and enzyme conformation. Carbohydr Res. 1986 Jun 1;149(1):13–21. doi: 10.1016/s0008-6215(00)90365-3. [DOI] [PubMed] [Google Scholar]
- Mason R. P., Moring J., Herbette L. G. A molecular model involving the membrane bilayer in the binding of lipid soluble drugs to their receptors in heart and brain. Int J Rad Appl Instrum B. 1990;17(1):13–33. doi: 10.1016/0883-2897(90)90004-k. [DOI] [PubMed] [Google Scholar]
- Mehta R. J., Nash C. H., Grappel S. F., Actor P. Aculeacin A resistant mutants of Candida albicans. J Antibiot (Tokyo) 1982 Jun;35(6):707–711. doi: 10.7164/antibiotics.35.707. [DOI] [PubMed] [Google Scholar]
- Mizoguchi J., Saito T., Mizuno K., Hayano K. On the mode of action of a new antifungal antibiotic, aculeacin A: inhibition of cell wall synthesis in Saccharomyces cerevisiae. J Antibiot (Tokyo) 1977 Apr;30(4):308–313. doi: 10.7164/antibiotics.30.308. [DOI] [PubMed] [Google Scholar]
- Mizuno K., Yagi A., Satoi S., Takada M., Hayashi M. Studies on aculeacin. I. Isolation and characterization of aculeacin A. J Antibiot (Tokyo) 1977 Apr;30(4):297–302. doi: 10.7164/antibiotics.30.297. [DOI] [PubMed] [Google Scholar]
- Pfaller M. A., Wey S., Gerarden T., Houston A., Wenzel R. P. Susceptibility of nosocomial isolates of Candida species to LY121019 and other antifungal agents. Diagn Microbiol Infect Dis. 1989 Jan-Feb;12(1):1–4. doi: 10.1016/0732-8893(89)90035-7. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Cabib E. Two forms of yeast glycogen synthetase and their role in glycogen accumulation. Proc Natl Acad Sci U S A. 1970 Jul;66(3):967–974. doi: 10.1073/pnas.66.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawistowska-Schröder E. T., Kerridge D., Perry H. Echinocandin inhibition of 1,3-beta-D-glucan synthase from Candida albicans. FEBS Lett. 1984 Jul 23;173(1):134–138. doi: 10.1016/0014-5793(84)81032-7. [DOI] [PubMed] [Google Scholar]
- Schmatz D. M., Romancheck M. A., Pittarelli L. A., Schwartz R. E., Fromtling R. A., Nollstadt K. H., Vanmiddlesworth F. L., Wilson K. E., Turner M. J. Treatment of Pneumocystis carinii pneumonia with 1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5950–5954. doi: 10.1073/pnas.87.15.5950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. E., Sesin D. F., Joshua H., Wilson K. E., Kempf A. J., Goklen K. A., Kuehner D., Gailliot P., Gleason C., White R. Pneumocandins from Zalerion arboricola. I. Discovery and isolation. J Antibiot (Tokyo) 1992 Dec;45(12):1853–1866. doi: 10.7164/antibiotics.45.1853. [DOI] [PubMed] [Google Scholar]
- Sperka-Gottlieb C. D., Hermetter A., Paltauf F., Daum G. Lipid topology and physical properties of the outer mitochondrial membrane of the yeast, Saccharomyces cerevisiae. Biochim Biophys Acta. 1988 Dec 22;946(2):227–234. doi: 10.1016/0005-2736(88)90397-5. [DOI] [PubMed] [Google Scholar]
- Taft C. S., Selitrennikoff C. P. Cilofungin inhibition of (1-3)-beta-glucan synthase: the lipophilic side chain is essential for inhibition of enzyme activity. J Antibiot (Tokyo) 1990 Apr;43(4):433–437. doi: 10.7164/antibiotics.43.433. [DOI] [PubMed] [Google Scholar]
- Taft C. S., Stark T., Selitrennikoff C. P. Cilofungin (LY121019) inhibits Candida albicans (1-3)-beta-D-glucan synthase activity. Antimicrob Agents Chemother. 1988 Dec;32(12):1901–1903. doi: 10.1128/aac.32.12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambias R. A., Hammond M. L., Heck J. V., Bartizal K., Trainor C., Abruzzo G., Schmatz D. M., Nollstadt K. M. Preparation and structure-activity relationships of simplified analogues of the antifungal agent cilofungin: a total synthesis approach. J Med Chem. 1992 Jul 24;35(15):2843–2855. doi: 10.1021/jm00093a018. [DOI] [PubMed] [Google Scholar]
- Zinser E., Sperka-Gottlieb C. D., Fasch E. V., Kohlwein S. D., Paltauf F., Daum G. Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. J Bacteriol. 1991 Mar;173(6):2026–2034. doi: 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]


