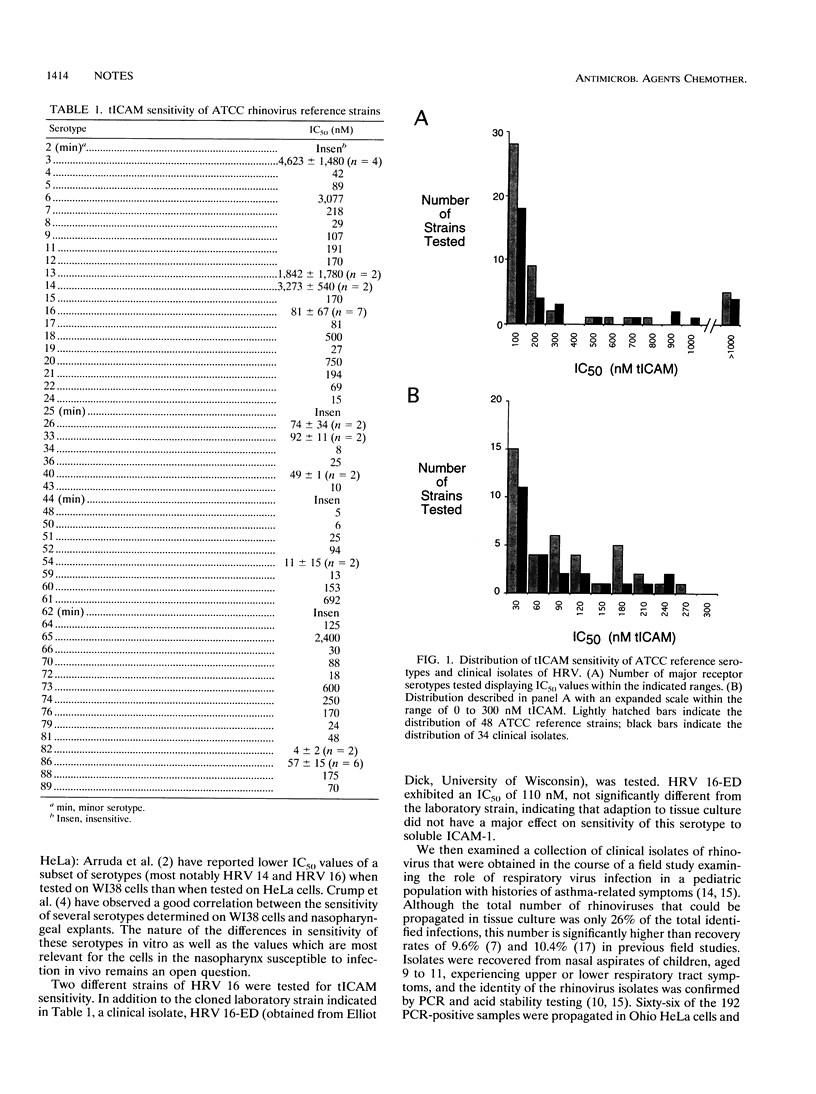
Abstract
The antiviral potency of soluble intercellular adhesion molecule-1 (ICAM-1; the major receptor for human rhinoviruses) was determined for a subset of American Type Culture Collection reference serotypes and field isolates of rhinovirus. The results indicate that soluble ICAM-1 exhibits a broad spectrum of activity against rhinoviruses and that field isolates have a sensitivity indistinguishable from that of laboratory strains.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andries K., Dewindt B., De Brabander M., Stokbroekx R., Janssen P. A. In vitro activity of R 61837, a new antirhinovirus compound. Arch Virol. 1988;101(3-4):155–167. doi: 10.1007/BF01310997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arruda E., Crump C. E., Marlin S. D., Merluzzi V. J., Hayden F. G. In vitro studies of the antirhinovirus activity of soluble intercellular adhesion molecule-1. Antimicrob Agents Chemother. 1992 Jun;36(6):1186–1191. doi: 10.1128/aac.36.6.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Callahan P. L., Long W. J. Isolation of a monoclonal antibody that blocks attachment of the major group of human rhinoviruses. J Virol. 1986 Jan;57(1):7–12. doi: 10.1128/jvi.57.1.7-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daar E. S., Li X. L., Moudgil T., Ho D. D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearden C., al-Nakib W., Andries K., Woestenborghs R., Tyrrell D. A. Drug resistant rhinoviruses from the nose of experimentally treated volunteers. Arch Virol. 1989;109(1-2):71–81. doi: 10.1007/BF01310519. [DOI] [PubMed] [Google Scholar]
- Fox J. P., Cooney M. K., Hall C. E., Foy H. M. Rhinoviruses in Seattle families, 1975-1979. Am J Epidemiol. 1985 Nov;122(5):830–846. doi: 10.1093/oxfordjournals.aje.a114166. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Davis G., Meyer A. M., Forte C. P., Yost S. C., Marlor C. W., Kamarck M. E., McClelland A. The major human rhinovirus receptor is ICAM-1. Cell. 1989 Mar 10;56(5):839–847. doi: 10.1016/0092-8674(89)90688-0. [DOI] [PubMed] [Google Scholar]
- Greve J. M., Forte C. P., Marlor C. W., Meyer A. M., Hoover-Litty H., Wunderlich D., McClelland A. Mechanisms of receptor-mediated rhinovirus neutralization defined by two soluble forms of ICAM-1. J Virol. 1991 Nov;65(11):6015–6023. doi: 10.1128/jvi.65.11.6015-6023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamparian V. V., Colonno R. J., Cooney M. K., Dick E. C., Gwaltney J. M., Jr, Hughes J. H., Jordan W. S., Jr, Kapikian A. Z., Mogabgab W. J., Monto A. A collaborative report: rhinoviruses--extension of the numbering system from 89 to 100. Virology. 1987 Jul;159(1):191–192. doi: 10.1016/0042-6822(87)90367-9. [DOI] [PubMed] [Google Scholar]
- Heinz B. A., Rueckert R. R., Shepard D. A., Dutko F. J., McKinlay M. A., Fancher M., Rossmann M. G., Badger J., Smith T. J. Genetic and molecular analyses of spontaneous mutants of human rhinovirus 14 that are resistant to an antiviral compound. J Virol. 1989 Jun;63(6):2476–2485. doi: 10.1128/jvi.63.6.2476-2485.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivey-Hoyle M., Culp J. S., Chaikin M. A., Hellmig B. D., Matthews T. J., Sweet R. W., Rosenberg M. Envelope glycoproteins from biologically diverse isolates of immunodeficiency viruses have widely different affinities for CD4. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):512–516. doi: 10.1073/pnas.88.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston S. L., Sanderson G., Pattemore P. K., Smith S., Bardin P. G., Bruce C. B., Lambden P. R., Tyrrell D. A., Holgate S. T. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J Clin Microbiol. 1993 Jan;31(1):111–117. doi: 10.1128/jcm.31.1.111-117.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Staunton D. E., Springer T. A., Stratowa C., Sommergruber W., Merluzzi V. J. A soluble form of intercellular adhesion molecule-1 inhibits rhinovirus infection. Nature. 1990 Mar 1;344(6261):70–72. doi: 10.1038/344070a0. [DOI] [PubMed] [Google Scholar]
- Monto A. S., Cavallaro J. J. The Tecumseh study of respiratory illness. IV. Prevalence of rhinovirus serotypes, 1966-1969. Am J Epidemiol. 1972 Nov;96(5):352–360. doi: 10.1093/oxfordjournals.aje.a121466. [DOI] [PubMed] [Google Scholar]
- Staunton D. E., Merluzzi V. J., Rothlein R., Barton R., Marlin S. D., Springer T. A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell. 1989 Mar 10;56(5):849–853. doi: 10.1016/0092-8674(89)90689-2. [DOI] [PubMed] [Google Scholar]


