Abstract
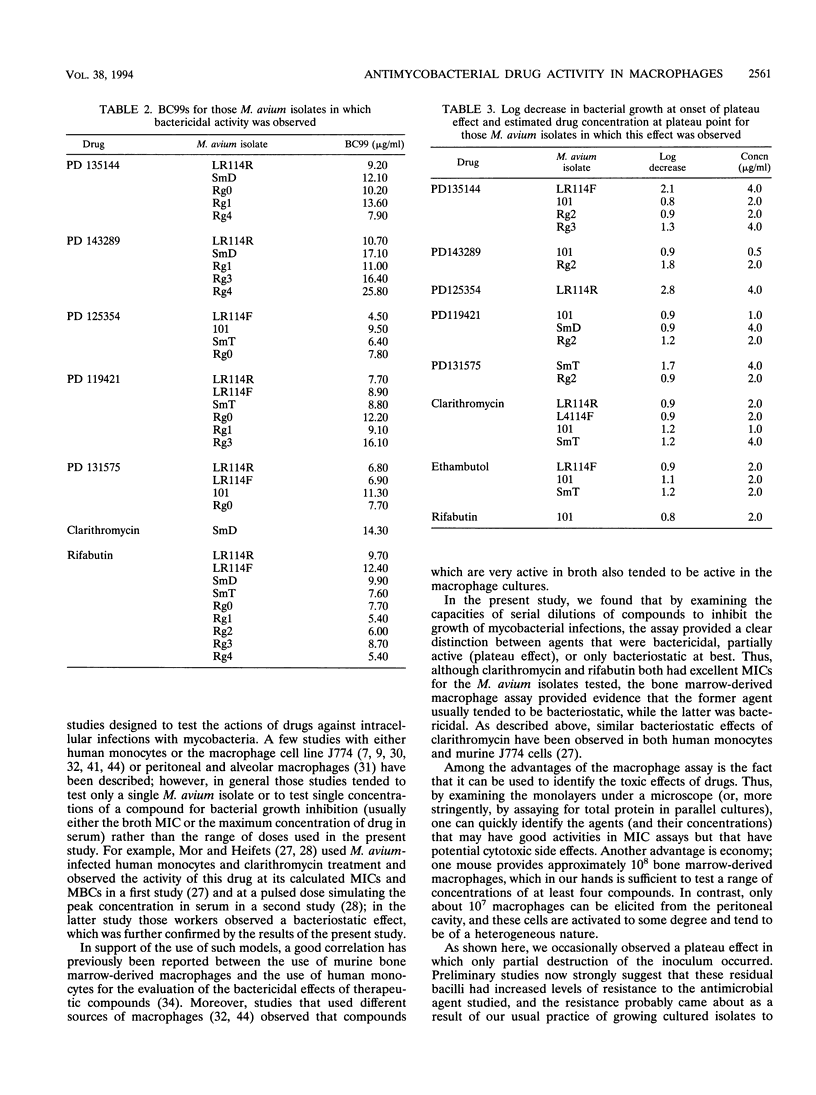
Even though the macrophage is the host cell for the intracellular bacterial parasite Mycobacterium avium, macrophages have undergone only limited evaluation as models for determining the capacities of antimycobacterial drugs to inhibit the growth of M. avium within this relevant intracellular environment. In the present study, we demonstrated that a panel of M. avium isolates could actively infect homogeneous monolayers of murine bone marrow-derived macrophages. A number of established and experimental antimycobacterial drugs were then added to these cultures at a range of concentrations, and their effects on the numbers of surviving bacilli were determined 8 days later. By plotting such numbers versus drug concentrations it was then possible to clearly distinguish between compounds with bactericidal activity (such as rifabutin and PD 125354) and those with bacteriostatic effects (such as clarithromycin), even though several of these compounds had very similar MICs. In addition, an estimate of the potential therapeutic efficiency of each drug could be made by determining the concentration needed to destroy an arbitrary percentage of the inoculum (in this case, the bactericidal concentration destroying 99% of the inoculum). Such values were considerably in excess of the MICs and may more realistically reflect the concentrations in serum required to effectively reduce the bacterial burden in vivo.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appelberg R., Orme I. M. Effector mechanisms involved in cytokine-mediated bacteriostasis of Mycobacterium avium infections in murine macrophages. Immunology. 1993 Nov;80(3):352–359. [PMC free article] [PubMed] [Google Scholar]
- Belisle J. T., Klaczkiewicz K., Brennan P. J., Jacobs W. R., Jr, Inamine J. M. Rough morphological variants of Mycobacterium avium. Characterization of genomic deletions resulting in the loss of glycopeptidolipid expression. J Biol Chem. 1993 May 15;268(14):10517–10523. [PubMed] [Google Scholar]
- Benson C. A., Ellner J. J. Mycobacterium avium complex infection and AIDS: advances in theory and practice. Clin Infect Dis. 1993 Jul;17(1):7–20. doi: 10.1093/clinids/17.1.7. [DOI] [PubMed] [Google Scholar]
- Bermudez L. E., Inderlied C., Young L. S. Stimulation with cytokines enhances penetration of azithromycin into human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2625–2629. doi: 10.1128/aac.35.12.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Activities of amikacin, roxithromycin, and azithromycin alone or in combination with tumor necrosis factor against Mycobacterium avium complex. Antimicrob Agents Chemother. 1988 Aug;32(8):1149–1153. doi: 10.1128/aac.32.8.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y., Perronne C., Truffot-Pernot C., Grosset J., Vilde J. L., Pocidalo J. J. Activities of WIN-57273, minocycline, clarithromycin, and 14-hydroxy-clarithromycin against Mycobacterium avium complex in human macrophages. Antimicrob Agents Chemother. 1992 Oct;36(10):2104–2107. doi: 10.1128/aac.36.10.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Dahl R., Ross E., May M. H. Evidence that vesicles containing living, virulent Mycobacterium tuberculosis or Mycobacterium avium in cultured human macrophages are not acidic. Infect Immun. 1991 May;59(5):1823–1831. doi: 10.1128/iai.59.5.1823-1831.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowle A. J., Elkins N., May M. H. Effectiveness of ofloxacin against Mycobacterium tuberculosis and Mycobacterium avium, and rifampin against M. tuberculosis in cultured human macrophages. Am Rev Respir Dis. 1988 May;137(5):1141–1146. doi: 10.1164/ajrccm/137.5.1141. [DOI] [PubMed] [Google Scholar]
- Crowle A. J., Tsang A. Y., Vatter A. E., May M. H. Comparison of 15 laboratory and patient-derived strains of Mycobacterium avium for ability to infect and multiply in cultured human macrophages. J Clin Microbiol. 1986 Nov;24(5):812–821. doi: 10.1128/jcm.24.5.812-821.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dautzenberg B. Clinical trials in Mycobacterium avium therapy: lessons to take home. Res Microbiol. 1994 Mar-Apr;145(3):197–206. doi: 10.1016/0923-2508(94)90018-3. [DOI] [PubMed] [Google Scholar]
- David H. L. Basis for lack of drug susceptibility of atypical mycobacteria. Rev Infect Dis. 1981 Sep-Oct;3(5):878–884. doi: 10.1093/clinids/3.5.878. [DOI] [PubMed] [Google Scholar]
- Ellner J. J., Goldberger M. J., Parenti D. M. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J Infect Dis. 1991 Jun;163(6):1326–1335. doi: 10.1093/infdis/163.6.1326. [DOI] [PubMed] [Google Scholar]
- Furney S. K., Skinner P. S., Roberts A. D., Appelberg R., Orme I. M. Capacity of Mycobacterium avium isolates to grow well or poorly in murine macrophages resides in their ability to induce secretion of tumor necrosis factor. Infect Immun. 1992 Oct;60(10):4410–4413. doi: 10.1128/iai.60.10.4410-4413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D. Determination of in vitro susceptibility of mycobacteria to ansamycin. Am Rev Respir Dis. 1985 Sep;132(3):710–711. doi: 10.1164/arrd.1985.132.3.710. [DOI] [PubMed] [Google Scholar]
- Heifets L. B., Iseman M. D., Lindholm-Levy P. J. Combinations of rifampin or rifabutine plus ethambutol against Mycobacterium avium complex. Bactericidal synergistic, and bacteriostatic additive or synergistic effects. Am Rev Respir Dis. 1988 Mar;137(3):711–715. doi: 10.1164/ajrccm/137.3.711. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Havlik J. A., Ellis D. A., Kennedy E., Fann S. A., Dubois R. E., Thompson S. E. Survival of patients with acquired immune deficiency syndrome and disseminated Mycobacterium avium complex infection with and without antimycobacterial chemotherapy. Am Rev Respir Dis. 1991 Sep;144(3 Pt 1):557–559. doi: 10.1164/ajrccm/144.3_Pt_1.557. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Selik R. M. The epidemiology of disseminated nontuberculous mycobacterial infection in the acquired immunodeficiency syndrome (AIDS). Am Rev Respir Dis. 1989 Jan;139(1):4–7. doi: 10.1164/ajrccm/139.1.4. [DOI] [PubMed] [Google Scholar]
- Inderlied C. B., Kemper C. A., Bermudez L. E. The Mycobacterium avium complex. Clin Microbiol Rev. 1993 Jul;6(3):266–310. doi: 10.1128/cmr.6.3.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inderlied C. B., Young L. S., Yamada J. K. Determination of in vitro susceptibility of Mycobacterium avium complex isolates to antimycobacterial agents by various methods. Antimicrob Agents Chemother. 1987 Nov;31(11):1697–1702. doi: 10.1128/aac.31.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Li J. Y., Wang S., Pearson A. J., Chang K., Jacobs M. R., Bajaksouzian S., Ellner J. J. In vitro anti-Mycobacterium avium activities of quinolones: predicted active structures and mechanistic considerations. Antimicrob Agents Chemother. 1994 Aug;38(8):1794–1802. doi: 10.1128/aac.38.8.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Wang S., Jacobs M. R., Bajaksouzian S., Edmonds K., Ellner J. J. Anti-Mycobacterium avium activity of quinolones: in vitro activities. Antimicrob Agents Chemother. 1993 Sep;37(9):1799–1806. doi: 10.1128/aac.37.9.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopman G., Wang S., Jacobs M. R., Ellner J. J. Anti-Mycobacterium avium activity of quinolones: structure-activity relationship studies. Antimicrob Agents Chemother. 1993 Sep;37(9):1807–1815. doi: 10.1128/aac.37.9.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leysen D. C., Haemers A., Pattyn S. R. Mycobacteria and the new quinolones. Antimicrob Agents Chemother. 1989 Jan;33(1):1–5. doi: 10.1128/aac.33.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masur H. Recommendations on prophylaxis and therapy for disseminated Mycobacterium avium complex disease in patients infected with the human immunodeficiency virus. Public Health Service Task Force on Prophylaxis and Therapy for Mycobacterium avium Complex. N Engl J Med. 1993 Sep 16;329(12):898–904. doi: 10.1056/NEJM199309163291228. [DOI] [PubMed] [Google Scholar]
- Mor N., Heifets L. Inhibition of intracellular growth of Mycobacterium avium by one pulsed exposure of infected macrophages to clarithromycin. Antimicrob Agents Chemother. 1993 Jun;37(6):1380–1382. doi: 10.1128/aac.37.6.1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor N., Heifets L. MICs and MBCs of clarithromycin against Mycobacterium avium within human macrophages. Antimicrob Agents Chemother. 1993 Jan;37(1):111–114. doi: 10.1128/aac.37.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S. D., Cameron D. W., Gordin F. M., Sullam P. M., Cohn D. L., Chaisson R. E., Eron L. J., Sparti P. D., Bihari B., Kaufman D. L. Two controlled trials of rifabutin prophylaxis against Mycobacterium avium complex infection in AIDS. N Engl J Med. 1993 Sep 16;329(12):828–833. doi: 10.1056/NEJM199309163291202. [DOI] [PubMed] [Google Scholar]
- Perronne C., Gikas A., Truffot-Pernot C., Grosset J., Pocidalo J. J., Vilde J. L. Activities of clarithromycin, sulfisoxazole, and rifabutin against Mycobacterium avium complex multiplication within human macrophages. Antimicrob Agents Chemother. 1990 Aug;34(8):1508–1511. doi: 10.1128/aac.34.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal V. K., Gangadharam P. R., Iseman M. D. Effect of rifabutin on the phagocytosis and intracellular growth of Mycobacterium intracellulare in mouse resident and activated peritoneal and alveolar macrophages. Am Rev Respir Dis. 1987 Aug;136(2):334–337. doi: 10.1164/ajrccm/136.2.334. [DOI] [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V. Extracellular and intracellular activities of clarithromycin used alone and in association with ethambutol and rifampin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Mar;35(3):462–470. doi: 10.1128/aac.35.3.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi N., Labrousse V., Goh K. S., De Sousa J. P. Antimycobacterial spectrum of sparfloxacin and its activities alone and in association with other drugs against Mycobacterium avium complex growing extracellularly and intracellularly in murine and human macrophages. Antimicrob Agents Chemother. 1991 Dec;35(12):2473–2480. doi: 10.1128/aac.35.12.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Sato K., Tomioka H. Comparative in vitro and in vivo activity of rifabutin and rifampicin against Mycobacterium avium complex. Tubercle. 1988 Sep;69(3):187–192. doi: 10.1016/0041-3879(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Saito H., Tomioka H. Susceptibilities of transparent, opaque, and rough colonial variants of Mycobacterium avium complex to various fatty acids. Antimicrob Agents Chemother. 1988 Mar;32(3):400–402. doi: 10.1128/aac.32.3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiratsuchi H., Johnson J. L., Toba H., Ellner J. J. Strain- and donor-related differences in the interaction of Mycobacterium avium with human monocytes and its modulation by interferon-gamma. J Infect Dis. 1990 Oct;162(4):932–938. doi: 10.1093/infdis/162.4.932. [DOI] [PubMed] [Google Scholar]
- Sturgill-Koszycki S., Schlesinger P. H., Chakraborty P., Haddix P. L., Collins H. L., Fok A. K., Allen R. D., Gluck S. L., Heuser J., Russell D. G. Lack of acidification in Mycobacterium phagosomes produced by exclusion of the vesicular proton-ATPase. Science. 1994 Feb 4;263(5147):678–681. doi: 10.1126/science.8303277. [DOI] [PubMed] [Google Scholar]
- Truffot-Pernot C., Ji B., Grosset J. Effect of pH on the in vitro potency of clarithromycin against Mycobacterium avium complex. Antimicrob Agents Chemother. 1991 Aug;35(8):1677–1678. doi: 10.1128/aac.35.8.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodley C. L., David H. L. Effect of temperature on the rate of the transparent to opaque colony type transition in Mycobacterium avium. Antimicrob Agents Chemother. 1976 Jan;9(1):113–119. doi: 10.1128/aac.9.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M. In vitro activity of antimicrobial agents against the Mycobacterium avium complex inside macrophages from HIV1-infected individuals: the link to clinical response to treatment? Res Microbiol. 1992 May;143(4):411–419. doi: 10.1016/0923-2508(92)90055-s. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Kirihara J., Sanders C., Nassos P., Hadley W. K. Antimicrobial synergism against Mycobacterium avium complex strains isolated from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1988 Sep;32(9):1392–1395. doi: 10.1128/aac.32.9.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987 Oct;31(10):1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Sanders C. A., Hadley W. K. Killing by antimycobacterial agents of AIDS-derived strains of Mycobacterium avium complex inside cells of the mouse macrophage cell line J774. Am Rev Respir Dis. 1989 Nov;140(5):1198–1203. doi: 10.1164/ajrccm/140.5.1198. [DOI] [PubMed] [Google Scholar]
- Young L. S. Mycobacterium avium complex infection. J Infect Dis. 1988 May;157(5):863–867. doi: 10.1093/infdis/157.5.863. [DOI] [PubMed] [Google Scholar]




