Abstract
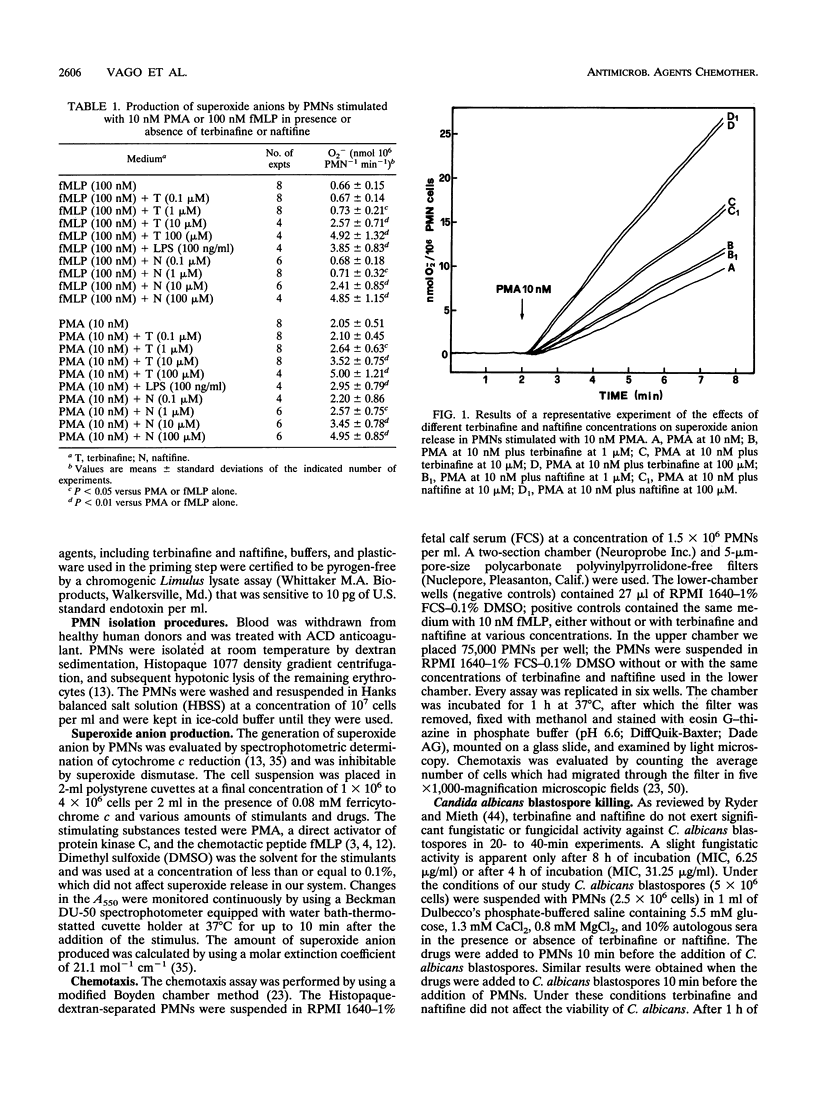
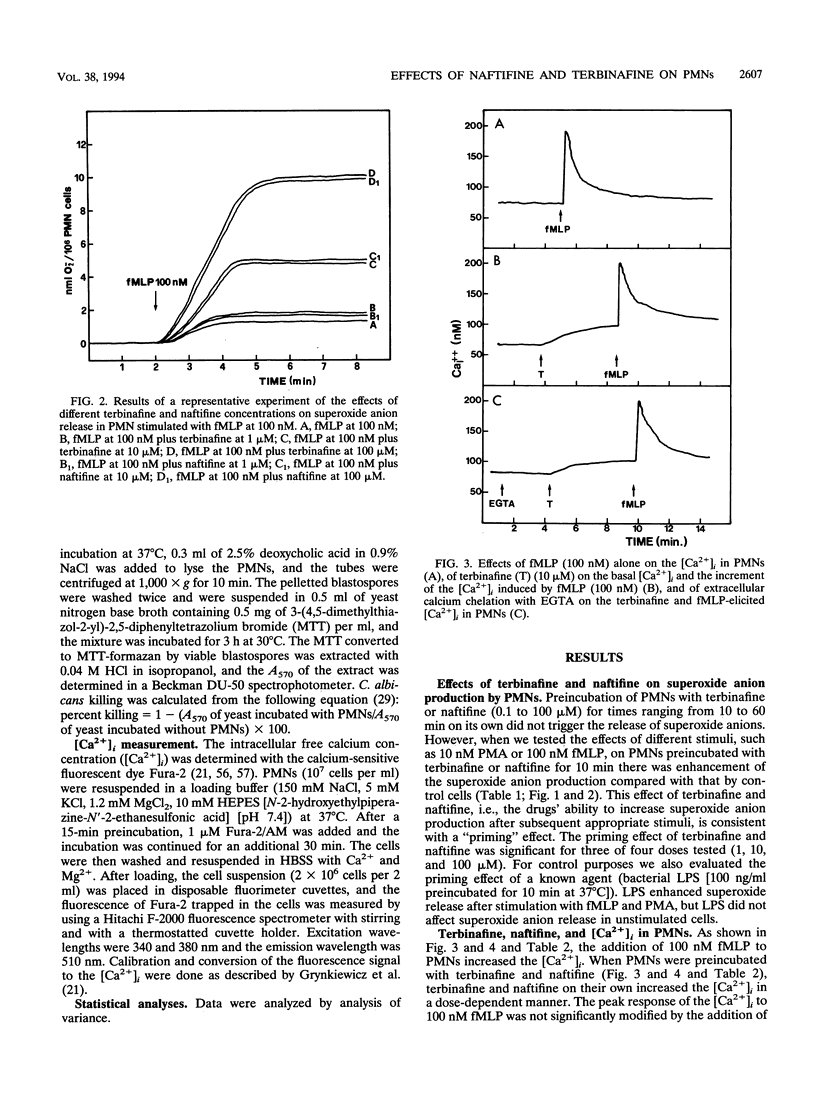
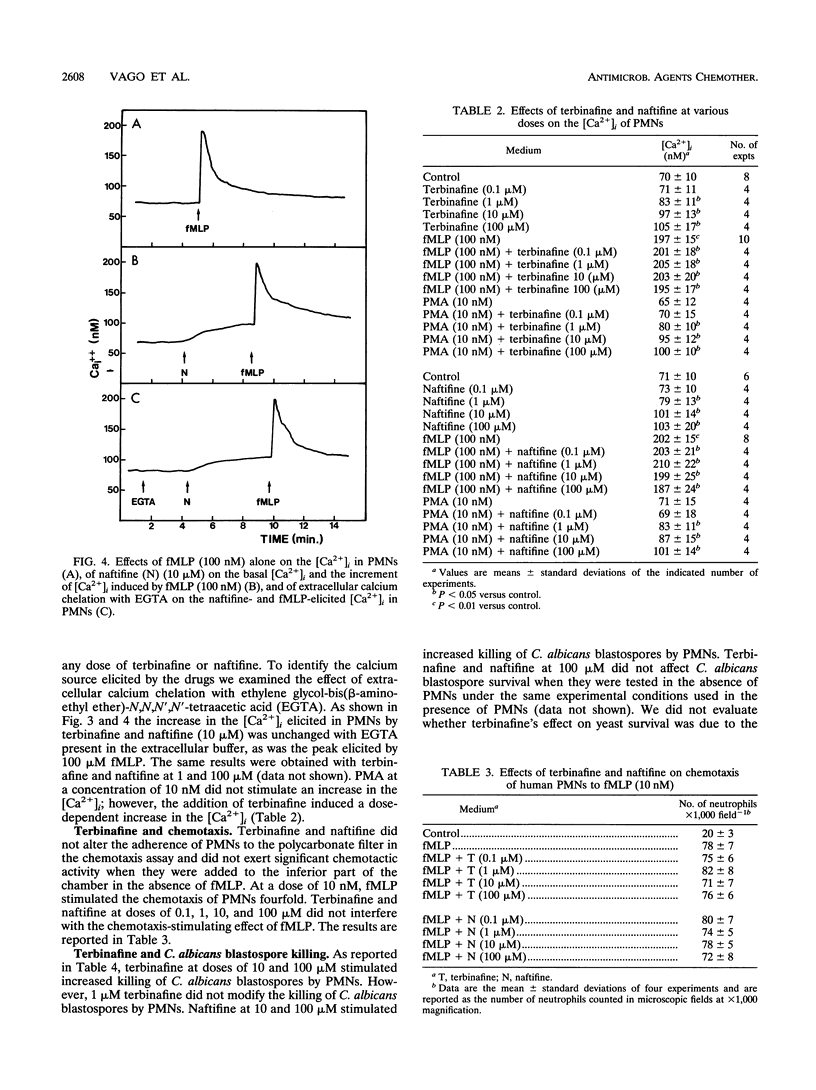
Many antimycotic agents negatively affect the natural immune response. Typically, these drugs impair polymorphonuclear leukocyte (PMN) production of superoxide anion, chemotaxis, or the killing of pathogens. Allylamines are a new class of antimycotic compounds with a new mechanism of antifungal action, i.e., inhibition of the fungal squalene epoxidase. The trial that we describe aimed to evaluate the effects of two allylamines, terbinafine and naftifine, on selected functions of PMNs, i.e., superoxide anion production, chemotaxis, and killing of Candida albicans blastospores. Terbinafine and naftifine on their own did not affect superoxide anion production when they were added to PMNs. When PMNs were preincubated with allylamines and were then stimulated by N-formyl-Met-Leu-Phe or phorbol 12-myristate 13-acetate, superoxide anion production was increased (priming effect). Since intracellular free calcium (Ca2+i) is involved in the control of superoxide anion production, we evaluated the effects of the allylamines on the Ca2+i concentration ([Ca2+]i). In the presence of terbinafine or naftifine, the [Ca2+]i increased in a dose-dependent manner; the source of Ca2+i was not extracellular since it was not affected by extracellular calcium chelation with ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid. In the presence of terbinafine or naftifine, chemotaxis of PMNs was not impaired. Terbinafine and naftifine slightly but significantly increased the killing of C. albicans blastospores (P < 0.05 at 10 and 100 microM). In conclusion, in contrast to imidazole-like drugs, the allylamine antimycotic compounds terbinafine and naftifine enhance selected functions of PMNs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo G. K., Fromtling R. A., Turnbull T. A., Giltinan D. M. Effects of bifonazole, fluconazole, itraconazole, and terbinafine on the chemiluminescence response of immune cells. J Antimicrob Chemother. 1987 Jul;20(1):61–68. doi: 10.1093/jac/20.1.61. [DOI] [PubMed] [Google Scholar]
- Anséhn S., Granström S., Höjer H., Nilsson L., Akesson E., Lundin A., Thore A. In-vitro effects of Candida albicans of amphotericin B combined with other antibiotics. Preliminary observations. Scand J Infect Dis Suppl. 1976;(9):62–66. [PubMed] [Google Scholar]
- Babior B. M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984 Nov;64(5):959–966. [PubMed] [Google Scholar]
- Becker E. L. The short and happy life of neutrophil activation. J Leukoc Biol. 1990 Apr;47(4):378–389. doi: 10.1002/jlb.47.4.378. [DOI] [PubMed] [Google Scholar]
- Beggs W. H., Sarosi G. A., Andrews F. A. Synergistic action of amphotericin B and rifampin on Candida albicans. Am Rev Respir Dis. 1974 Nov;110(5):671–673. doi: 10.1164/arrd.1974.110.5.671. [DOI] [PubMed] [Google Scholar]
- Berliner S., Weinberger M., Ben-Bassat M., Lavie G., Weinberger A., Giller S., Pinkhas J. Amphotericin B causes aggregation of neutrophils and enhances pulmonary leukostasis. Am Rev Respir Dis. 1985 Sep;132(3):602–605. doi: 10.1164/arrd.1985.132.3.602. [DOI] [PubMed] [Google Scholar]
- Bint A. J. Esters of penicillins--are they hepatotoxic? J Antimicrob Chemother. 1980 Nov;6(6):697–699. doi: 10.1093/jac/6.6.697. [DOI] [PubMed] [Google Scholar]
- Björkstén B., Ray C., Quie P. G. Inhibition of human neutrophil chemotaxis and chemiluminescence by amphotericin B. Infect Immun. 1976 Jul;14(1):315–317. doi: 10.1128/iai.14.1.315-317.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. A., Ingraham L. M., Allen J., Oseas R. S., Baehner R. L. Amphotericin-B promotes leukocyte aggregation of nylon-wool-fiber-treated polymorphonuclear leukocytes. Blood. 1981 Sep;58(3):518–523. [PubMed] [Google Scholar]
- Chan C. K., Balish E. Inhibition of granulocyte phagocytosis of Candida albicans by amphotericin B. Can J Microbiol. 1978 Apr;24(4):363–364. doi: 10.1139/m78-061. [DOI] [PubMed] [Google Scholar]
- Chunn C. J., Starr P. R., Gilbert D. N. Neutrophil toxicity of amphotericin B. Antimicrob Agents Chemother. 1977 Aug;12(2):226–230. doi: 10.1128/aac.12.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A. The human neutrophil respiratory burst oxidase. J Infect Dis. 1990 Jun;161(6):1140–1147. doi: 10.1093/infdis/161.6.1140. [DOI] [PubMed] [Google Scholar]
- Dallegri F., Ballestrero A., Frumento G., Patrone F. Augmentation of neutrophil-mediated erythrocyte lysis by cells derived in vitro from human monocytes. Blood. 1987 Dec;70(6):1743–1749. [PubMed] [Google Scholar]
- Diamond R. D., Lyman C. A., Wysong D. R. Disparate effects of interferon-gamma and tumor necrosis factor-alpha on early neutrophil respiratory burst and fungicidal responses to Candida albicans hyphae in vitro. J Clin Invest. 1991 Feb;87(2):711–720. doi: 10.1172/JCI115050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. E., Jr, Morrison J., Henderson D. K., Montgomerie J. Z. Combined effect of amphotericin B and rifampin on Candida species. Antimicrob Agents Chemother. 1980 Mar;17(3):484–487. doi: 10.1128/aac.17.3.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas B., Dobozy A. The effect of ketoconazole on the phagocytosis and intracellular killing of Candida albicans by polymorphonuclear granulocytes. Mykosen. 1983 Jan;26(1):22–26. doi: 10.1111/j.1439-0507.1983.tb03932.x. [DOI] [PubMed] [Google Scholar]
- Fujita N. K., Edwards J. E., Jr Combined in vitro effect of amphotericin B and rifampin on Cryptococcus neoformans. Antimicrob Agents Chemother. 1981 Jan;19(1):196–198. doi: 10.1128/aac.19.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gergely P., Nékám K., Láng I., Kalmár L., González-Cabello R., Perl A. Ketoconazole in vitro inhibits mitogen-induced blastogenesis, antibody-dependent cellular cytotoxicity, natural killer activity and random migration of human leukocytes. Immunopharmacology. 1984 Jun;7(3-4):167–170. doi: 10.1016/0162-3109(84)90033-x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Guthrie L. A., McPhail L. C., Henson P. M., Johnston R. B., Jr Priming of neutrophils for enhanced release of oxygen metabolites by bacterial lipopolysaccharide. Evidence for increased activity of the superoxide-producing enzyme. J Exp Med. 1984 Dec 1;160(6):1656–1671. doi: 10.1084/jem.160.6.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvath L., Falk W., Leonard E. J. Rapid quantitation of neutrophil chemotaxis: use of a polyvinylpyrrolidone-free polycarbonate membrane in a multiwell assembly. J Immunol Methods. 1980;37(1):39–45. doi: 10.1016/0022-1759(80)90179-9. [DOI] [PubMed] [Google Scholar]
- Hauser W. E., Jr, Remington J. S. Effect of antibiotics on the immune response. Am J Med. 1982 May;72(5):711–716. doi: 10.1016/0002-9343(82)90534-4. [DOI] [PubMed] [Google Scholar]
- Heeres J., Backx L. J., Van Cutsem J. Antimycotic azoles. 7. Synthesis and antifungal properties of a series of novel triazol-3-ones. J Med Chem. 1984 Jul;27(7):894–900. doi: 10.1021/jm00373a015. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Warnock D. W., Richardson M. D., Douglas C. J. In-vitro effect of itraconazole, ketoconazole and amphotericin B on the phagocytic and candidacidal function of human neutrophils. J Antimicrob Chemother. 1986 Jul;18(1):83–91. doi: 10.1093/jac/18.1.83. [DOI] [PubMed] [Google Scholar]
- Klein J. B., Payne V., Schepers T. M., McLeish K. R. Bacterial lipopolysaccharide enhances polymorphonuclear leukocyte function independent of changes in intracellular calcium. Inflammation. 1990 Oct;14(5):599–611. doi: 10.1007/BF00914279. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Diamond R. D. A rapid colorimetric assay of fungal viability with the tetrazolium salt MTT. J Infect Dis. 1985 Nov;152(5):938–945. doi: 10.1093/infdis/152.5.938. [DOI] [PubMed] [Google Scholar]
- Lew P. D., Monod A., Waldvogel F. A., Dewald B., Baggiolini M., Pozzan T. Quantitative analysis of the cytosolic free calcium dependency of exocytosis from three subcellular compartments in intact human neutrophils. J Cell Biol. 1986 Jun;102(6):2197–2204. doi: 10.1083/jcb.102.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S. H., Medoff G., Kobayashi G. S. Effects of amphotericin B on macrophages and their precursor cells. Antimicrob Agents Chemother. 1977 Jan;11(1):154–160. doi: 10.1128/aac.11.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohr K. M., Snyderman R. Amphotericin B alters the affinity and functional activity of the oligopeptide chemotactic factor receptor on human polymorphonuclear leukocytes. J Immunol. 1982 Oct;129(4):1594–1599. [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Marmer D. J., Fields B. T., Jr, France G. L., Steele R. W. Ketoconazole, amphotericin B, and amphotericin B methyl ester: comparative in vitro and in vivo toxicological effects on neutrophil function. Antimicrob Agents Chemother. 1981 Nov;20(5):660–665. doi: 10.1128/aac.20.5.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maródi L., Schreiber S., Anderson D. C., MacDermott R. P., Korchak H. M., Johnston R. B., Jr Enhancement of macrophage candidacidal activity by interferon-gamma. Increased phagocytosis, killing, and calcium signal mediated by a decreased number of mannose receptors. J Clin Invest. 1993 Jun;91(6):2596–2601. doi: 10.1172/JCI116498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshulam T., Diamond R. D., Lyman C. A., Wysong D. R., Melnick D. A. Temporal association of calcium mobilization, inositol trisphosphate generation, and superoxide anion release by human neutrophils activated by serum opsonized and nonopsonized particulate stimuli. Biochem Biophys Res Commun. 1988 Jan 29;150(2):532–539. doi: 10.1016/0006-291x(88)90426-3. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Couchot K. R. Effects of sublethal concentrations of amphotericin B on Candida albicans. J Infect Dis. 1986 Oct;154(4):665–669. doi: 10.1093/infdis/154.4.665. [DOI] [PubMed] [Google Scholar]
- Pallister C. J., Johnson E. M., Warnock D. W., Elliot P. J., Reeves D. F. In-vitro effects of liposome-encapsulated amphotericin B (AmBisome) and amphotericin B-deoxycholate (Fungizone) on the phagocytic and candidacidal function of human polymorphonuclear leucocytes. J Antimicrob Chemother. 1992 Sep;30(3):313–320. doi: 10.1093/jac/30.3.313. [DOI] [PubMed] [Google Scholar]
- Pallister C. J., Warnock D. W. Effect of antimicrobial and antineoplastic drugs alone and in combination on the phagocytic and candidacidal function of human polymorphonuclear leucocytes. J Antimicrob Chemother. 1989 Jan;23(1):87–94. doi: 10.1093/jac/23.1.87. [DOI] [PubMed] [Google Scholar]
- Petranyi G., Ryder N. S., Stütz A. Allylamine derivatives: new class of synthetic antifungal agents inhibiting fungal squalene epoxidase. Science. 1984 Jun 15;224(4654):1239–1241. doi: 10.1126/science.6547247. [DOI] [PubMed] [Google Scholar]
- Ponce E., Pechère J. C. Activity of amphotericin B and intraconazole against intraphagocytic Candida albicans. Eur J Clin Microbiol Infect Dis. 1990 Oct;9(10):738–744. doi: 10.1007/BF02184686. [DOI] [PubMed] [Google Scholar]
- Roilides E., Walsh T. J., Rubin M., Venzon D., Pizzo P. A. Effects of antifungal agents on the function of human neutrophils in vitro. Antimicrob Agents Chemother. 1990 Feb;34(2):196–201. doi: 10.1128/aac.34.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan-Kelly B., Ferrante A., Thong Y. H. Modification of polymorphonuclear leucocyte function by imidazoles. Int J Immunopharmacol. 1984;6(4):389–393. doi: 10.1016/0192-0561(84)90059-6. [DOI] [PubMed] [Google Scholar]
- Ryder N. S., Mieth H. Allylamine antifungal drugs. Curr Top Med Mycol. 1992;4:158–188. doi: 10.1007/978-1-4612-2762-5_6. [DOI] [PubMed] [Google Scholar]
- Sample A. K., Czuprynski C. J. Priming and stimulation of bovine neutrophils by recombinant human interleukin-1 alpha and tumor necrosis factor alpha. J Leukoc Biol. 1991 Feb;49(2):107–115. doi: 10.1002/jlb.49.2.107. [DOI] [PubMed] [Google Scholar]
- Schaude M., Ackerbauer H., Mieth H. Inhibitory effect of antifungal agents on germ tube formation in Candida albicans. Mykosen. 1987 Jun;30(6):281–287. doi: 10.1111/j.1439-0507.1987.tb03980.x. [DOI] [PubMed] [Google Scholar]
- Schaumann R. F., Shah P. M. Effect of amphotericin B alone or in combination with rifampicin on phagocytosis of Candida species by human polymorphonuclear leukocytes. Methods Find Exp Clin Pharmacol. 1992 Dec;14(10):753–758. [PubMed] [Google Scholar]
- Schuster I. The interaction of representative members from two classes of antimycotics--the azoles and the allylamines--with cytochromes P-450 in steroidogenic tissues and liver. Xenobiotica. 1985 Jun;15(6):529–546. doi: 10.3109/00498258509045027. [DOI] [PubMed] [Google Scholar]
- Senior D. S., Shaw J. T. In vitro effects of fluconazole (UK-49,858) and ketoconazole on mouse lymphocyte proliferation and on Candida blastospore destruction by human polymorphonuclear leukocytes. Int J Immunopharmacol. 1988;10(2):169–173. doi: 10.1016/0192-0561(88)90092-6. [DOI] [PubMed] [Google Scholar]
- Senior R. M., Hinek A., Griffin G. L., Pipoly D. J., Crouch E. C., Mecham R. P. Neutrophils show chemotaxis to type IV collagen and its 7S domain and contain a 67 kD type IV collagen binding protein with lectin properties. Am J Respir Cell Mol Biol. 1989 Dec;1(6):479–487. doi: 10.1165/ajrcmb/1.6.479. [DOI] [PubMed] [Google Scholar]
- Solomon B. A., Lee W. L., Geen S. C., Suntharalingam K., Fikrig S. M., Shalita A. R. Modification of neutrophil functions by naftifine. Br J Dermatol. 1993 Apr;128(4):393–398. doi: 10.1111/j.1365-2133.1993.tb00197.x. [DOI] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Mandell G. L. Lipid complexing decreases amphotericin B inflammatory activation of human neutrophils compared with that of a desoxycholate-suspended preparation of amphotericin B (Fungizone). Antimicrob Agents Chemother. 1992 Jan;36(1):39–45. doi: 10.1128/aac.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G. W., Carper H. T., Mandell G. L. Pentoxifylline modulates activation of human neutrophils by amphotericin B in vitro. Antimicrob Agents Chemother. 1992 Feb;36(2):408–416. doi: 10.1128/aac.36.2.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supapidhayakul S. R., Kizlaitis L. R., Andersen B. R. Stimulation of human and canine neutrophil metabolism by amphotericin B. Antimicrob Agents Chemother. 1981 Feb;19(2):284–289. doi: 10.1128/aac.19.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targett G. A. Chemotherapy and the immune response in parasitic infections. Parasitology. 1985 Apr;90(Pt 4):661–673. doi: 10.1017/s003118200005229x. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. New calcium indicators and buffers with high selectivity against magnesium and protons: design, synthesis, and properties of prototype structures. Biochemistry. 1980 May 27;19(11):2396–2404. doi: 10.1021/bi00552a018. [DOI] [PubMed] [Google Scholar]
- Van 't Wout J. W., Meynaar I., Linde I., Poell R., Mattie H., Van Furth R. Effect of amphotericin B, fluconazole and itraconazole on intracellular Candida albicans and germ tube development in macrophages. J Antimicrob Chemother. 1990 May;25(5):803–811. doi: 10.1093/jac/25.5.803. [DOI] [PubMed] [Google Scholar]
- Van der Auwera P., Meunier F. In-vitro effects of cilofungin (LY121019), amphotericin B and amphotericin B-deoxycholate on human polymorphonuclear leucocytes. J Antimicrob Chemother. 1989 Nov;24(5):747–763. doi: 10.1093/jac/24.5.747. [DOI] [PubMed] [Google Scholar]
- Vuddhakul V., Mai G. T., McCormack J. G., Seow W. K., Thong Y. H. Suppression of neutrophil and lymphoproliferative responses in vitro by itraconazole but not fluconazole. Int J Immunopharmacol. 1990;12(6):639–645. doi: 10.1016/0192-0561(90)90101-r. [DOI] [PubMed] [Google Scholar]
- Vuddhakul V., McCormack J. G., Seow W. K., Thong Y. H. Effects of the newer antifungal agents (bifonazole, ICI 195, 739 and amorolfin) on in vitro phagocytic, lymphocytic and natural-killer cell responses. Int J Immunopharmacol. 1989;11(7):817–828. doi: 10.1016/0192-0561(89)90136-7. [DOI] [PubMed] [Google Scholar]
- Wilson E., Thorson L., Speert D. P. Enhancement of macrophage superoxide anion production by amphotericin B. Antimicrob Agents Chemother. 1991 May;35(5):796–800. doi: 10.1128/aac.35.5.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysong D. R., Lyman C. A., Diamond R. D. Independence of neutrophil respiratory burst oxidant generation from the early cytosolic calcium response after stimulation with unopsonized Candida albicans hyphae. Infect Immun. 1989 May;57(5):1499–1505. doi: 10.1128/iai.57.5.1499-1505.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K., Masuda M., Matsuoka T., Yamazaki M., Komiyama A., Akabane T., Murata K. Miconazole and amphotericin B alter polymorphonuclear leukocyte functions and membrane fluidity in similar fashions. Antimicrob Agents Chemother. 1988 Dec;32(12):1864–1868. doi: 10.1128/aac.32.12.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rensburg C. E., Anderson R., Jooné G., van der Merwe M. F., Eftychis H. A. The effects of ketoconazole on cellular and humoral immune functions. J Antimicrob Chemother. 1983 Jan;11(1):49–55. doi: 10.1093/jac/11.1.49. [DOI] [PubMed] [Google Scholar]



