Abstract
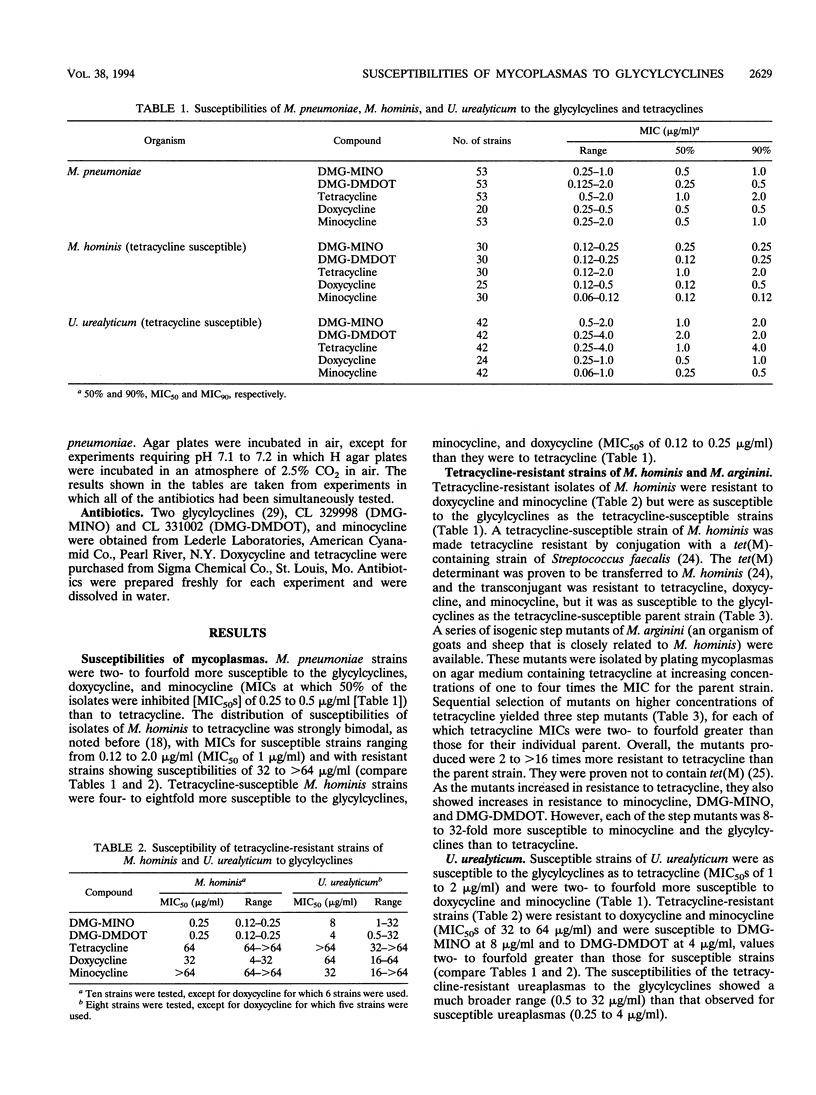
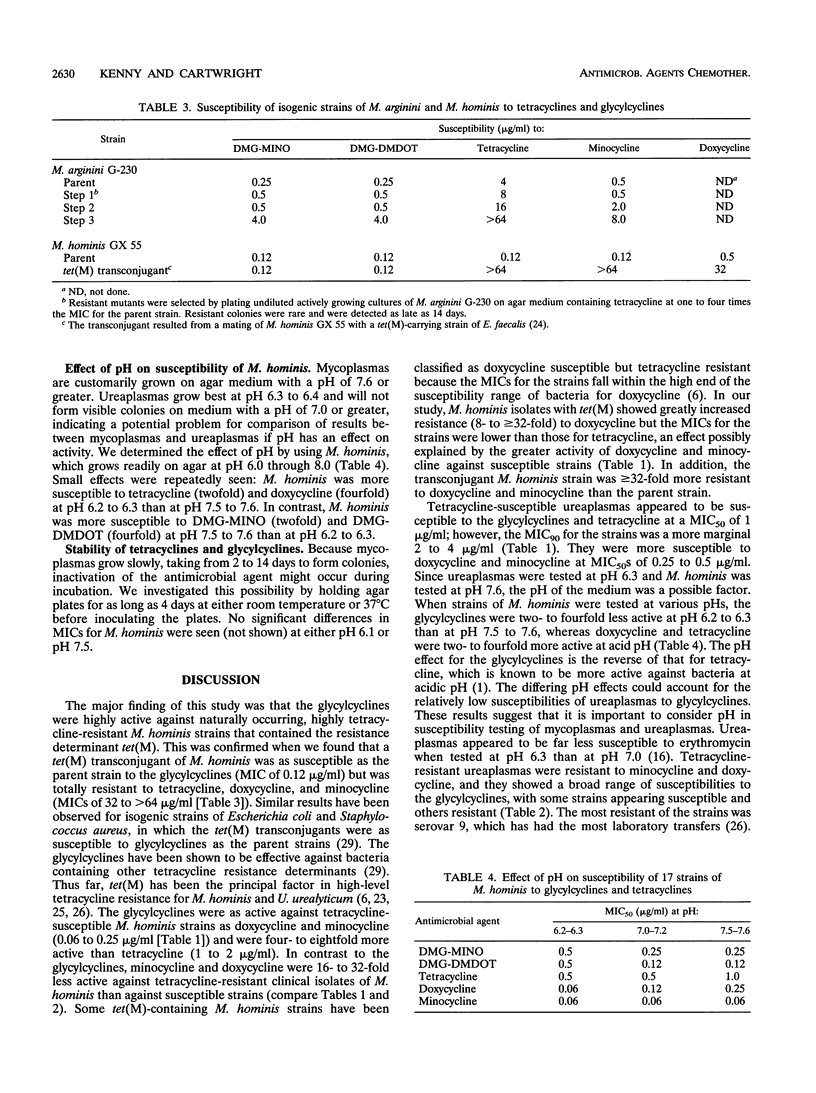
The glycylcyclines are new tetracycline derivatives that include the N,N-dimethylglycylamido derivative of minocycline (DMG-MINO) and the N,N-dimethylglycylamido derivative of 6-demethyl-6-deoxytetracycline (DMG-DMDOT). The susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to DMG-MINO, DMG-DMDOT, tetracycline, doxycycline, and minocycline were determined by the agar dilution method. The glycylcyclines with MICs at which 50% of the isolates are inhibited of 0.25 to 0.5 micrograms/ml for M. pneumoniae were two- to fourfold more active than tetracycline and had the same activity as minocycline and doxycycline. Tetracycline-susceptible M. hominis strains were four- to eightfold more susceptible to the glycylcyclines (0.12 to 0.25 micrograms/ml) than to tetracycline. Strains of M. hominis known to be resistant to tetracycline, doxycycline, and minocycline because of the tet(M) determinant were as susceptible to the glycylcyclines as the tetracycline-susceptible strains. For tetracycline-susceptible U. urealyticum strains, the glycylcyclines showed the same activity as tetracycline (MICs at which 50% of the isolates are inhibited of 1 to 2 micrograms/ml). Tetracycline-resistant strains of U. urealyticum were resistant to doxycycline and minocycline and showed variable susceptibility to the glycylcyclines (range, 0.5 to 32 micrograms/ml). In view of the increasing resistance of M. hominis and U. urealyticum strains to tetracyclines, the glycylcyclines have promise, pending assessment of their pharmacokinetic and safety profiles.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai S., Gohara Y., Kuwano K., Kawashima T. Antimycoplasmal activities of new quinolones, tetracyclines, and macrolides against Mycoplasma pneumoniae. Antimicrob Agents Chemother. 1992 Jun;36(6):1322–1324. doi: 10.1128/aac.36.6.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barile M. F., DelGiudice R. A., Carski T. R., Gibbs C. J., Morris J. A. Isolation and characterization of Mycoplasma arginini: spec. nov. Proc Soc Exp Biol Med. 1968 Nov;129(2):489–494. doi: 10.3181/00379727-129-33351. [DOI] [PubMed] [Google Scholar]
- Bauriaud R., Séror C., Lareng M. B., Lefèvre J. C. Sensibilité in vitro aux antibiotiques des mycoplasmes génitaux isolés à Toulouse. Etude de nouvelles molécules (macrolides et quinolones). Pathol Biol (Paris) 1992 May;40(5):479–482. [PubMed] [Google Scholar]
- Bebear C., Cantet P., Renaudin H., Quentin C. Activité comparée de la minocycline et doxycycline sur les mycoplasmes pathogènes pour l'homme. Pathol Biol (Paris) 1985 Jun;33(5 Pt 2):577–580. [PubMed] [Google Scholar]
- Blanchard A., Crabb D. M., Dybvig K., Duffy L. B., Cassell G. H. Rapid detection of tetM in Mycoplasma hominis and Ureaplasma urealyticum by PCR: tetM confers resistance to tetracycline but not necessarily to doxycycline. FEMS Microbiol Lett. 1992 Aug 15;74(2-3):277–281. doi: 10.1016/0378-1097(92)90442-q. [DOI] [PubMed] [Google Scholar]
- Cummings M. C., McCormack W. M. Increase in resistance of Mycoplasma hominis to tetracyclines. Antimicrob Agents Chemother. 1990 Dec;34(12):2297–2299. doi: 10.1128/aac.34.12.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliopoulos G. M., Wennersten C. B., Cole G., Moellering R. C. In vitro activities of two glycylcyclines against gram-positive bacteria. Antimicrob Agents Chemother. 1994 Mar;38(3):534–541. doi: 10.1128/aac.38.3.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalil N., Gilchrist C., Taylor-Robinson D. Factors influencing the in-vitro sensitivity of Ureaplasma urealyticum to tetracyclines. J Antimicrob Chemother. 1989 Mar;23(3):341–345. doi: 10.1093/jac/23.3.341. [DOI] [PubMed] [Google Scholar]
- Jao R. L. Susceptibility of Mycoplasma pneumoniae to 21 antibiotics in vitro. Am J Med Sci. 1967 Jun;253(6):639–650. doi: 10.1097/00000441-196706000-00001. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Effect of pH, inoculum size, and incubation time on the susceptibility of Ureaplasma urealyticum to erythromycin in vitro. Clin Infect Dis. 1993 Aug;17 (Suppl 1):S215–S218. doi: 10.1093/clinids/17.supplement_1.s215. [DOI] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Susceptibilities of Mycoplasma hominis and Ureaplasma urealyticum to two new quinolones, sparfloxacin and WIN 57273. Antimicrob Agents Chemother. 1991 Jul;35(7):1515–1516. doi: 10.1128/aac.35.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Susceptibilities of Mycoplasma hominis, Mycoplasma pneumoniae, and Ureaplasma urealyticum to a new quinolone, OPC 17116. Antimicrob Agents Chemother. 1993 Aug;37(8):1726–1727. doi: 10.1128/aac.37.8.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E., Cartwright F. D. Susceptibility of Mycoplasma pneumoniae to several new quinolones, tetracycline, and erythromycin. Antimicrob Agents Chemother. 1991 Mar;35(3):587–589. doi: 10.1128/aac.35.3.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny G. E., Hooton T. M., Roberts M. C., Cartwright F. D., Hoyt J. Susceptibilities of genital mycoplasmas to the newer quinolones as determined by the agar dilution method. Antimicrob Agents Chemother. 1989 Jan;33(1):103–107. doi: 10.1128/aac.33.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laborde M. Etude de la sensibilité aux antibiotiques de 34 souches de mycoplasmes a grandes colonies. Pathol Biol (Paris) 1977 Oct;25(8):541–546. [PubMed] [Google Scholar]
- Mårdh P. A. Human respiratory tract infections with mycoplasmas and their in vitro susceptibility to tetracyclines and some other antibiotics. Chemotherapy. 1975;21 (Suppl 1):47–57. doi: 10.1159/000221891. [DOI] [PubMed] [Google Scholar]
- Neu H. C. The crisis in antibiotic resistance. Science. 1992 Aug 21;257(5073):1064–1073. doi: 10.1126/science.257.5073.1064. [DOI] [PubMed] [Google Scholar]
- Roberts M. C., Kenny G. E. Conjugal transfer of transposon Tn916 from Streptococcus faecalis to Mycoplasma hominis. J Bacteriol. 1987 Aug;169(8):3836–3839. doi: 10.1128/jb.169.8.3836-3839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Kenny G. E. Dissemination of the tetM tetracycline resistance determinant to Ureaplasma urealyticum. Antimicrob Agents Chemother. 1986 Feb;29(2):350–352. doi: 10.1128/aac.29.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts M. C., Koutsky L. A., Holmes K. K., LeBlanc D. J., Kenny G. E. Tetracycline-resistant Mycoplasma hominis strains contain streptococcal tetM sequences. Antimicrob Agents Chemother. 1985 Jul;28(1):141–143. doi: 10.1128/aac.28.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J. A., Stemke G. W., Maclellan S. G., Taylor D. E. Characterization of tetracycline-resistant strains of Ureaplasma urealyticum. J Antimicrob Chemother. 1988 Mar;21(3):319–332. doi: 10.1093/jac/21.3.319. [DOI] [PubMed] [Google Scholar]
- Rylander M., Hallander H. O. In vitro comparison of the activity of doxycycline, tetracycline, erythromycin and a new macrolide, CP 62993, against Mycoplasma pneumoniae, Mycoplasma hominis and Ureaplasma urealyticum. Scand J Infect Dis Suppl. 1988;53:12–17. [PubMed] [Google Scholar]
- Speer B. S., Shoemaker N. B., Salyers A. A. Bacterial resistance to tetracycline: mechanisms, transfer, and clinical significance. Clin Microbiol Rev. 1992 Oct;5(4):387–399. doi: 10.1128/cmr.5.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa R. T., Petersen P. J., Jacobus N. V., Sum P. E., Lee V. J., Tally F. P. In vitro and in vivo antibacterial activities of the glycylcyclines, a new class of semisynthetic tetracyclines. Antimicrob Agents Chemother. 1993 Nov;37(11):2270–2277. doi: 10.1128/aac.37.11.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites K. B., Duffy L. B., Schmid T., Crabb D., Pate M. S., Cassell G. H. In vitro susceptibilities of Mycoplasma pneumoniae, Mycoplasma hominis, and Ureaplasma urealyticum to sparfloxacin and PD 127391. Antimicrob Agents Chemother. 1991 Jun;35(6):1181–1185. doi: 10.1128/aac.35.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites K. B., Figarola T. A., Schmid T., Crabb D. M., Duffy L. B., Simecka J. W. Comparison of agar versus broth dilution techniques for determining antibiotic susceptibilities of Ureaplasma urealyticum. Diagn Microbiol Infect Dis. 1991 May-Jun;14(3):265–271. doi: 10.1016/0732-8893(91)90041-d. [DOI] [PubMed] [Google Scholar]
- Wise R., Andrews J. M. In vitro activities of two glycylcyclines. Antimicrob Agents Chemother. 1994 May;38(5):1096–1102. doi: 10.1128/aac.38.5.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]


