Abstract
Autoimmune insulin-dependent diabetes mellitus (IDDM) occurs spontaneously in mice-bearing transgenes encoding the influenza hemagglutinin under the control of the rat insulin promoter and a T cell receptor specific for an hemagglutinin peptide associated with I-Ed. Such “double transgenic” mice expressing wild-type or targeted IL-4Rα genes were examined for the onset of IDDM. Eight of 11 mice homozygous for wild-type IL-4Rα were hyperglycemic by 8 weeks of age, whereas only 1 of 16 mice homozygous for the targeted allele were hyperglycemic at this time. Most 1L-4Rα−/− mice remained normoglycemic to 36 weeks of age. Although only 10% of double transgenic mice homozygous for the wild-type IL-4Rα allele survived to 30 weeks, 80% of mice homozygous for the targeted allele did so. Heterozygous mice displayed an intermediate frequency of diabetes. Even as late as 270 days of age, mice homozygous for the targeted allele had no insulitis or only peri-insulitis. Thus, the inability to respond to IL-4 and/or IL-13 protects mice against IDDM in this model of autoimmunity.
Many tissue-specific autoimmune diseases are mediated by the induction of autoantigen-specific T cells. These cells are believed to cause tissue damage through the production of cytokines, through direct lysis of cells expressing self-antigens, or through the induction of inflammatory responses (1 -3). Examples of autoimmune diseases caused by the action of TH2 cells have been reported (4, 5), but this appears to be a less common mechanism of tissue-damaging autoimmunity. Indeed, some TH1-type autoimmune diseases can be prevented, or are ameliorated, by treatment with IL-4 (6, 7), which may either block the development of a TH1 response or may directly, or indirectly, inhibit the activity of the major product of TH1 cells, IFN-γ. In other models, peptide administration, which may induce a TH2 cytokine burst, can arrest autoimmunity (8–10).
In experimental type I diabetes, it has been generally assumed that TH1 cells are important in launching β-cell damage and that destruction of these cells is completed by CD8 T cells. Indeed, IFN-γ-producing TH1 clones derived from CD4 cells isolated from the pancreas of nonobese diabetic (NOD) mice transfer the disease to young NOD mice (11–13). In NOD mice, anti-IFN-γ antibodies have been reported to block the onset of cyclophosphamide-induced diabetes (14) or of diabetes induced by splenocyte transfer (15). However, a targeted mutation in the IFN-γ gene has been reported to delay but not prevent the onset of diabetes in NOD mice (16). Although IFN-γR knockout NOD mice were reported to be protected from diabetes (17), more recent work has demonstrated such an effect only in cyclophosphamide-induced acceleration of diabetes; a second gene, linked to the IFN-γR, plays a role in the resistance originally noted (18). On the other hand, treatment of NOD mice with IL-4 protects against the development of diabetes (6), and transgenic NOD mice expressing IL-4 under the rat insulin promoter are protected (19). Thus, the pattern of expressed cytokines plays an important but complex role in the induction of diabetes in NOD mice.
IFN-γ plays a causative role in other transgenic models of type I diabetes. For example, transgenic mice in which the lymphocytic choriomeningitis virus (LCMV) nucleoprotein or glycoprotein is expressed under the control of the rat insulin promoter develop diabetes upon infection with LCMV (20, 21). IFN-γ−/− mice are protected against diabetes in this model (22). In addition, if mice expressing the LCMV nucleoprotein transgene also express an IFN-γ transgene under the control of the rat insulin promotor, they develop diabetes without LCMV infection (23).
A particularly interesting model of type I diabetes involves the expression of transgenes encoding a T cell receptor specific for a peptide derived from influenza virus hemagglutinin (TCR-HA) and for the hemagglutinin gene under the control of the rat insulin promoter (RIP-HA). These mice develop diabetes with variable penetrance (24). In our laboratory, 50–80% of double transgenic mice are hyperglycemic by 4–8 weeks of age (25).
Recent results suggest that presentation of HA to, and priming of, specific T cells depends upon dendritic cells that acquire HA that has presumably been produced by islet cells (26). Such primed CD4 T cells (together with CD8 cells) are then capable of causing peri- and pan-insulitis and inducing diabetes.
Because IL-4 opposes the priming of TH1 cells in vitro and administering IL-4 can be protective in other models of diabetes, we were interested in testing the effect of deleting the IL-4 receptor α chain (IL-4Rα) on the development of diabetes in TCR-HA, RIP-HA double transgenic mice. We prepared such mice that were either homozygous for the wild-type allele of the IL-4Rα, chain, or heterozygous or homozygous for the targeted gene. Here, we report that, contrary to expectation, the lack of the IL-4Rα chain exerted a very significant protective effect on the occurrence of type I diabetes in these mice.
Materials and Methods
Mice.
BALB/c mice-expressing transgenes encoding a TCR recognizing an immunodominant epitope of influenza PR8 virus hemagglutinin (HA110–120) in association with I-Ed (TCR-HA) were kindly provided by Harald von Boehmer (Dana-Farber Cancer Institute, Boston), and B10.D2 mice expressing the PR8 influenza virus HA transgene (RIP-HA) were kindly provided by David Lo (Scripps Research Institute, La Jolla, CA). IL-4Rα−/− mice (27) were bred in the National Institute of Allergy and Infectious Diseases Animal Facilities. TCR-HA+/−, RIP-HA+/−, IL-4Rα−/−, +/−, +/+ mice were produced in the Mount Sinai Animal Facilities by mating TCR-HA+/−, IL-4Rα+/− mice with RIP-HA+/−, IL-4Rα+/− mice.
Progeny were initially genotyped for the expression of TCR-HA and RIP-HA transgenes. Double transgenic mice then were genotyped for the targeted mutation in the IL-4Rα gene.
The following primers were used under standard conditions for PCR: for the TCR-HA transgene, forward primer 5′-TAGGAGAAAGCAATGGAGAC-3′ and reverse primer 5′-GTACCTGGTATAACACTCAG-3′; for the HA transgene, forward primer 5′-GTCCTACATTGTAGAAACA-3′ and reverse primer 5′-GTGACTGGGTGTATATTCT-3′; for the wild-type and mutated IL-4Rct genes, forward primer 5′-AATGTGACCTACAAGGAACC-3′ and reverse primer 5′-GGACTCCACTCACTCCAG-3′; and for the neomycin resistance gene, forward primer 5′-CTTGGGTGGAGAGGCTATTC-3′ and reverse primer 5′-AGGTGAGATGACAGGAGATC-3′.
Diabetes Monitoring and Histology.
Mice were bled at 2-week intervals beginning at 8 weeks of age. Some were killed for histological analysis at various times.
Plasma glucose concentrations were measured with a Precision QID blood glucose monitor (Medisense, Bedford, MA).
Histopathological Analysis.
Pancreas specimens were fixed in 10% buffered formalin solution and embedded in paraffin, and sections were cut in stairwise 7-μm sections. This procedure was carried out to exclude counting the same islet twice. Sections were stained with hematoxylin-eosin.
Statistics.
Significance of differences in plasma glucose concentrations between various groups of mice was determined by Student's t test; significance in survival among various groups was determined by the Kaplan–Meier test (28). Differences were considered to be statistically significant when P values were <0.05.
Results
Genotyping of TCR-HA, RIP-HA Mice.
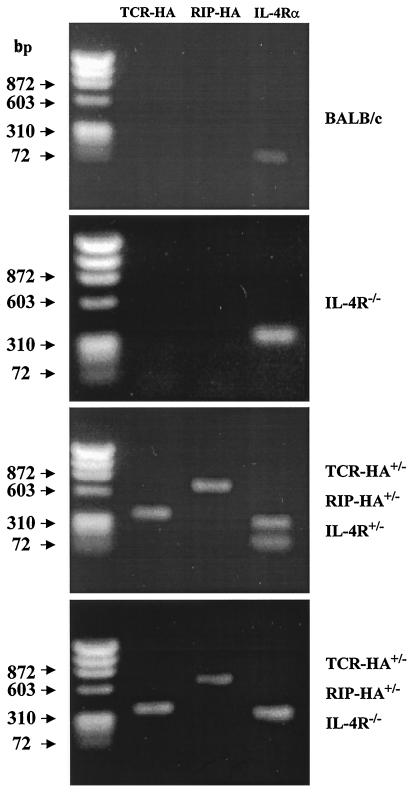
Progeny were obtained from the mating of TCR-HA+/−, IL-4Rα+/− mice with RIP-HA+/−, IL-4Rα+/− mice. Fig. 1 shows typical examples of genotyping. Genomic DNA from BALB/c mice yields a PCR product of 128 bp corresponding to the wild-type IL-4Rα gene, whereas the product obtained from IL-4Rα−/− DNA is 280 bp in length.
Figure 1.
Genotyping of mice by PCR. Genomic DNA was used as template. Primers for TCR-HA and RIP-HA transgenes and for wild-type and mutated IL-4Rα genes are as described in Materials and Methods. Lane 1, marker (DXI74 RF DNA/HaeIII fragments, 72–1353 bp); lane 2, TCR-HA; lane 3, RIP-HA; lane 4, IL-4Rα product.
Double Tg mice (TCR-HA+/−, RIP-HA+/−) display either the product of both wild and mutated gene (TCR-HA+/−, RIP-HA+/−, IL-4Rα +/−) of only mutated IL-4Rα gene (TCR-HA +/−, RIP-HA+/−, IL-4Rα−/−) or only of wild-type IL-4Rα gene (TCR-HA+/−, RIP-HA+/−, IL-4Rα+/+; data not shown). The specificity of the PCR reaction was confirmed by Southern blotting analysis using genomic DNA digested with EcoRI and hybridized with cDNA probes as previously described (27) (data not shown).
Frequency of Insulin-Dependent Diabetes Mellitus (IDDM).
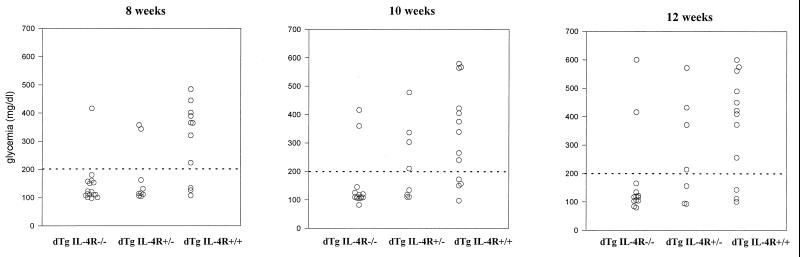
In our laboratory, many TCR-HA, RIP-HA double transgenic mice are hyperglycemic by 4 weeks of age (403 ± 117 mg glucose/dl) and are hypoinsulinemic (0.08 ± 0.03 ng/ml) at that time. We studied mice from the breeding scheme described above that were TCR-HA, RIP-HA at 8, 10, and 12 weeks of age. Eight of 11 of those homozygous for IL-4Rα were hyperglycemic at 8 weeks of age; the frequency of +/+ mice that were hyperglycemic changed little at 10 or 12 weeks of age (9 of 13 and 10 of 13, respectively). Double transgenic mice that were homozygous for the mutated IL-4Rα gene (−/−) mice showed a strikingly lower incidence of hyperglycemia (1 of 16 at 8 weeks, 2 of 14 at 10 weeks, and 2 of 13 at 12 weeks). The difference in incidence of hyperglycemia between the +/+ and −/− groups was highly significant (P < 0.003). The frequency of hyperglycemic mice among double transgenic IL-4Rα+/− mice at 8 weeks of age was only 2 of 10, significantly less than in the +/+ mice (P < 0.021). By 12 weeks of age, 4 of 7 of the +/− mice were hyperglycemic, suggesting that heterozygosity at the IL-4Rα locus delays, but does not prevent, the onset of diabetes (Fig. 2).
Figure 2.
Glycemia at 8–12 weeks of individual double transgenic mice.
Six of the double transgenic −/− mice that were normoglycemic at 12 weeks of age were followed for 24 weeks by bleeding every 2 weeks; these animals remained normoglycemic for the duration of the experiment.
Islet Histopathology.
Histopathology of TCR-HA+/−, RIP-HA+/− mice that were IL-4Rα+/− or −/− as well as that of their parental strains was examined. Double transgenic IL-4Rα +/− and −/− mice were divided into two groups: normoglycemic and hyperglycemic. As expected, BALB/c IL-4Rα−/−, B10.D2, RIP-HA+/− and BALB/c TCR-HA+/− mice were normoglycemic; their islets were of small size and showed no evidence of inflammation (Table 1). Furthermore, all TCR-HA+/−, RIP-HA+/− mice were hyperglycemic at 81 days of age, and all showed insulitis.
Table 1.
Correlation between glycemia and insulitis in double transgenic mice
| Genotype | Histopathology of pancreata
|
||||
|---|---|---|---|---|---|
| Age day | Insulitis
|
Normal (%) | # Islets counted | ||
| P | C | ||||
| (%) | |||||
| Parental strains | |||||
| BALB/c IL-4R −/− normoglycemic | 140 | 0 | 0 | 100 | 55 |
| B10.D2 RIP-HA +/− normoglycemic | 84 | 0 | 0 | 100 | 70 |
| BALB/c TCR-HA +/− normoglycemic | 140 | 0 | 0 | 100 | 68 |
| dTg mice | |||||
| TCR-HA +/−, RIP-HA +/−, IL-4R +/− normoglycemic | 84 | 0 | 0 | 100 | 27 |
| 84 | 0 | 0 | 100 | 20 | |
| 224 | 30 | 61 | 9 | 60 | |
| 224 | 7 | 79 | 14 | 14 | |
| 224 | 66 | 34 | 0 | 5 | |
| TCR-HA +/−, RIP-HA +/−, IL-4R +/− hyperglycemic | 70 | 13 | 83 | 3.6 | 138 |
| 77 | 7.1 | 78.6 | 14.3 | 14 | |
| 98 | 71 | 29 | 0 | 27 | |
| 196 | 5.5 | 94.5 | 0 | 28 | |
| 224 | 60 | 40 | 0 | 5 | |
| TCR-HA +/−, RIP-HA +/−, IL-4R −/− normoglycemic | 42 | 0 | 0 | 100 | 35 |
| 56 | 68 | 0 | 32 | 67 | |
| 56 | 0 | 0 | 100 | 35 | |
| 70 | 0 | 0 | 100 | 26 | |
| 98 | 0 | 0 | 100 | 24 | |
| 182 | 0 | 0 | 100 | 23 | |
| 182 | 0 | 0 | 100 | 22 | |
| 216 | 16 | 0 | 84 | 29 | |
| 216 | 8 | 0 | 92 | 20 | |
| 270 | 50 | 0 | 50 | 22 | |
| 270 | 0 | 0 | 100 | 39 | |
| TCR-HA +/−, RIP-HA +/−, IL-4R −/− | 98 | 71 | 29 | 0 | 42 |
| 140 | 63 | 37 | 0 | 19 | |
| 140 | 75 | 25 | 0 | 28 | |
P, peripheral-insulitis; C, pan-insulitis. Histological analysis of pancreata of eight hyperglycemic 84-day-old TCR-HA +/−, RIP-HA +/− double Tg mice showed 32 ± 21 pan-insulitis.
In Table 1, the histopathology of individual normoglycemic or hyperglycemic double transgenic IL-4Rα+/− or −/− mice, examined at various ages, is presented. Normoglycemic IL-4Rα−/− mice showed either no insulitis or a limited degree of peri-insulitis; the latter was observed principally in mice 216 days of age or older. Normoglycemic IL-4Rα+/− mice tended to have a somewhat greater degree of insulitis at 224 days of age; the three mice examined at that time had some islets that displayed pan-insulitis and others that showed peri-insulitis. All of the hyperglycemic IL-4Rα+/− and −/− mice displayed a substantial fraction of islets exhibiting pan-insulitis.
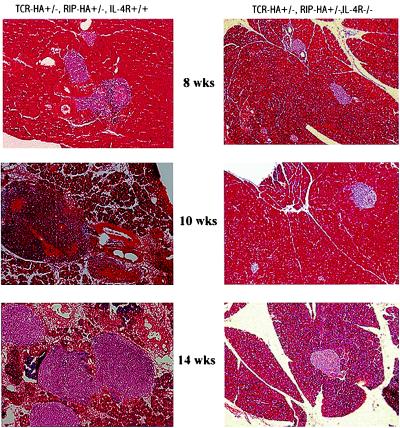
Fig. 3 illustrates the histopathology of pancreases of 8-, 10-, and 14-week-old double transgenic IL-4Rα+/+ and −/− mice. Whereas the islets of double transgenic IL-4R−/− mice are of normal size and do not exhibit inflammatory reactions, the islets of 8-week-old double transgenic IL-4R+/+ mice exhibit peri-insulitis; those of the 10- and 14-week-old +/+ mice exhibit pan-insulitis associated with a dramatic increase of the size caused by inflammatory reactions
Figure 3.
Pancreatic histology of pancreas of double transgenic IL-4Rα+/+ and IL-4Rα mice. Sections were stained with hematoxylin-eosin (×10).
Survival.
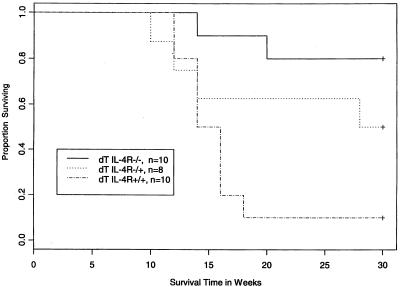
The survival of the double transgenic mice was followed over a 30-week period and analyzed by the Kaplan–Meier test (Fig. 4). The majority of the −/− mice survived, whereas the majority of +/+ mice died. The difference between these groups was highly significant (P = 0.00078). Although there was a trend for greater survival of the +/− mice than the +/+ mice, the difference was not statistically significant (P = 0.127). A larger sample will be needed to determine whether heterozygosity at the IL-4Rα locus is partially protective against death from IDDM.
Figure 4.
Kaplan–Meier survival curves of double transgenic mice.
Discussion
The IL-4Rα chain acts as the high affinity binding chain and the principal signaling chain for IL-4; it also acts as the signaling chain for IL-13, but in this case the IL-13Rα1 chain conveys the bulk of the cytokine specificity. Thus, IL-4Rα knockout mice are unresponsive to both IL-4 and IL-13 (29). The finding that the lack of IL-4Rα chain protects TCR-HA, RIP-HA double transgenic mice against insulitis, diabetes, and death implies that either IL-4 or IL-13 plays a role in the progression of this disease.
IL-4 can act at several loci in the immune response and thus might impact the development of autoimmune diabetes by more than one mechanism. It plays a critical role in the in vitro and in vivo induction of TH2 responses. Mice lacking IL-4 (30) as well as mice lacking IL-4Rα (27) or Stat6 (31) often make weak or absent TH2 responses. IL-4 and IL-13 may both act as effectors of allergic-type inflammation; IL-13 has often been the more important effector molecule (32). IL-5, a product of TH2 cells, plays an important role in mobilizing eosinophils from the bone marrow (33). Thus, if insulitis were mediated by “allergic” inflammatory responses, the absence of TH2 cytokines in IL-4Rα−/− mice could explain the protection of these animals from diabetes. Indeed, TH2 cells have been reported to transfer diabetes into NOD mice, but only if these animals are immunocompromised (5). The bulk of evidence implicates a TH1-driven type of immunopathology as the dominant force in the development of IDDM (14–23), arguing that the lack of IL-4Rα does not mediate its effect by diminishing TH2 effector functions.
Are their instances in which IL-4 contributes to the development of TH1 responses? IL-4 has been known for some time to play a role in the development of immature dendritic cells in vitro. Shortman and his colleagues (34) have recently reported that the production of the bioactive p70 form of IL-12 by freshly isolated and cultured mouse and human dendritic cells was strikingly enhanced by IL-4. If IL-4 plays a comparable role in vivo, it is possible that dendritic cells in IL-4Rα−/− mice would be less effective in polarizing CD4 responses toward the TH1 phenotype; indeed, such dendritic cells might act to tolerize rather than immunize naive cells.
There are some in vivo situations in which IL-4 actually appears to enhance TH1-like responses. Thus, IL-4-deficient BALB/c mice and BALB/c mice treated with anti-IL-4 antibody exhibit diminished contact hypersensitivity responses to dinitrofluorobenzine; IFN-γ production was diminished at the site of challenge (35, 36). IL-4−/− mice failed to develop contact hypersensitivity to trinitrochlorobenzine, although in this instance they did develop cells capable of producing TH1 cytokines (37). BALB/c mice, which are susceptible to progressive infection by the Leishmania major strain LV39, are partially protected by anti-IL-4 antibodies but not by knocking out either IL-4 or IL4Rα (38). This suggests that the small amount of IL-4 that failed to be neutralized by anti-IL-4 may be important in developing a protective TH1 response. Indeed, the administered monoclonal anti-IL-4 antibody did not appear to neutralize all IL-4, as these mice still developed elevation of serum IgE, although to a substantially lesser degree than mice not treated with anti-IL-4. Furthermore, Mencacci and colleagues have reported that IL-4−/− mice fail to develop a TH1 immune response against Candida (39).
These studies demonstrate a critical but largely unanticipated interplay between IL-4 and/or IL-13, the prototypic type 2 cytokines, and induction of a tissue-damaging TH1-mediated autoimmune response. The relevance of these observations for pathogenesis of spontaneous autoimmunity will require careful examination.
Acknowledgments
We thank Wendy Lou (Department of Biomathematical Science, Mount Sinai School of Medicine, New York) for assistance with statistical analyses. This work was supported in part by National Institutes of Health–National Institute of Allergy and Infectious Diseases Grant PO I-AI24671.
Abbreviations
- IDDM
insulin-dependent diabetes mellitus
- NOD
nonobese diabetic
- RIP
rat insulin promotor
- HA
hemagglutinin
- LCMV
lymphocytic choriomeningitis virus
- TCR
T cell receptor
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.230431397.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.230431397
References
- 1.Feldmann M, Brennan F M, Maini R. lnt Rev lmmunol. 1998;17:217–228. doi: 10.3109/08830189809084493. [DOI] [PubMed] [Google Scholar]
- 2.Theofilopoulos A N. lmmunol Today. 1995;l6:90–159. [Google Scholar]
- 3.Green E A, Flavell R A. Immunity. 2000;12:459–469. doi: 10.1016/s1074-7613(00)80198-3. [DOI] [PubMed] [Google Scholar]
- 4.Suri-Payer E, Amar A Z, McHugh R, Natarajan K, Margulies D H, Shevach E M. Eur J Immunol. 1999;29:669–677. doi: 10.1002/(SICI)1521-4141(199902)29:02<669::AID-IMMU669>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 5.Pakala S V, Kurrer M O, Katz J D. J Exp Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rapoport M J, Jaramillo A, Zipris D, Lazarus A H, Serreze D V, Leiter E H, Cyopick P, Danska J S, Delovitch T L. J Exp Med. 1993;178:87–99. doi: 10.1084/jem.178.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tominaga Y, Nagata M, Yasuda H, Okamoto N, Arisawa K, Moriyama H, Miki M, Yokono K, Kasuga M. Clin Immunol Immunopathol. 1998;86:209–218. doi: 10.1006/clin.1997.4471. [DOI] [PubMed] [Google Scholar]
- 8.Tisch R, Wang B, Serreze D V. J Immunol. 1999;163:1178–1187. [PubMed] [Google Scholar]
- 9.Polanski M, Melican N S, Zhang J, Weiner H L. J Autoimmun. 1997;10:339–346. doi: 10.1006/jaut.1997.0148. [DOI] [PubMed] [Google Scholar]
- 10.Maron R, Melican N S, Weiner H L. J Autoimmun. 1999;12:251–258. doi: 10.1006/jaut.1999.0278. [DOI] [PubMed] [Google Scholar]
- 11.Bradley L M, Asension V C, Schioetz L K, Harbertson J, Krahl T, Patstone G, Woolf N, Campbell I L, Sarvetnick N. J Immunol. 1999;162:2511–2120. [PubMed] [Google Scholar]
- 12.Zekzer D, Wong F S, Ayalon O, Millet I, Altieri M, Shintani S, Solimena M, Sherwin R S. J Clin Invest. 1998;101:68–73. doi: 10.1172/JCI119878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakala S V, Kurrer M O, Katz D J. J Exp Med. 1997;186:299–306. doi: 10.1084/jem.186.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Debray-Sachs M, Carnaud C, Boitard C, Cohen H, Gresser I, Bedossa P, Bach J-F. J Autoimmun. 1991;4:237–248. doi: 10.1016/0896-8411(91)90021-4. [DOI] [PubMed] [Google Scholar]
- 15.Campbell I L, Kay T W H, Oxbrow L, Harrison L C. J Clin Invest. 1991;87:739–742. doi: 10.1172/JCI115055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hultgren B, Huang X, Dybdal N, Stewart T A. Diabetes. 1996;45:812–817. doi: 10.2337/diab.45.6.812. [DOI] [PubMed] [Google Scholar]
- 17.Wang B, Andre I, Gonzalez A, Katz J D, Aguet M, Benoist C, Mathis D. Proc Natl Acad Sci USA. 1997;94:13844–13849. doi: 10.1073/pnas.94.25.13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kanagawa O, Xu G, Tevaarwerk A, Vaupel B A. J Immunol. 2000;164:3919–3923. doi: 10.4049/jimmunol.164.7.3919. [DOI] [PubMed] [Google Scholar]
- 19.Gallichan W S, Balasa B, Davies J D, Sarvetnick N. J Immunol. 1999;163:1696–1703. [PubMed] [Google Scholar]
- 20.Oldstone M B, Nerenberg M, Southern P, Lewicki H. Cell. 1991;65:319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- 21.Ohashi P S, Oehen S, Buerki K, Pircher H, Ohashi C T, Odermatt B, Malissen B, Zinkernagel R M, Hengartner H. Cell. 1991;65:305–317. doi: 10.1016/0092-8674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- 22.von Herrath M G, Oldstone M B. J Exp Med. 1997;185:531–539. doi: 10.1084/jem.185.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee M S, von Herrath M, Reiser H, Oldstone M B, Sarvetnick N. J Clin Invest. 1995;95:486–492. doi: 10.1172/JCI117689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarukhan A, Lanoue A, Franzke A, Brousse N, Buer J, von Boehmer H. EMBO J. 1998;17:71–80. doi: 10.1093/emboj/17.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarukhan A, Lechner O, von Boehmer H. Eur J Immunol. 1999;29:3410–3416. doi: 10.1002/(SICI)1521-4141(199910)29:10<3410::AID-IMMU3410>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 26.Radu D L, Brumeanu T D, McEvoy R C, Bona C A, Casares S. Autoimmunity. 1999;30:199–207. doi: 10.3109/08916939908993801. [DOI] [PubMed] [Google Scholar]
- 27.Noben-Trauth N, Shultz L D, Brombacher F, Urban J F, Gu H, Paul W E. Proc Natl Acad Sci USA. 1997;94:10838–10843. doi: 10.1073/pnas.94.20.10838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosner B. Fundamentals of Biostatistics. 4th Ed. Belmont, CA: Wadsworth; 1995. pp. 609–614. [Google Scholar]
- 29.Nelms K, Keegan A D, Zamorano J, Ryan J J, Paul W E. Annu Rev Immunol. 1999;17:701–738. doi: 10.1146/annurev.immunol.17.1.701. [DOI] [PubMed] [Google Scholar]
- 30.Kopf M, Le Gros G, Bachmann M, Lamers M C, Bluethmann H, Kohler G. Nature (London) 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 31.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Nature (London) 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 32.Chomarat P, Banchereau J. Int Rev Immunol. 1998;17:1–52. doi: 10.3109/08830189809084486. [DOI] [PubMed] [Google Scholar]
- 33.Karlen S, De Boer M L, Lipscombe R J, Lutz W, Mordvinov V A, Sanderson C J. Int Rev Immunol. 1998;16:227–247. doi: 10.3109/08830189809042996. [DOI] [PubMed] [Google Scholar]
- 34.Hochrein, H., O'Keeffe, M., Luft, T., Vandenabeele, S., Maraskovsky, E. & Shortman, K. (2000) J. Exp. Med., in press. [DOI] [PMC free article] [PubMed]
- 35.Nagai H, Ueda Y, Ochi T, Hirano Y, Tanaka H, Inagaki N, Kawada K. Br J Pharmacol. 2000;129:299–306. doi: 10.1038/sj.bjp.0703054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Traidl C, Jugert F, Krieg T, Merk H, Hunzelmann N. J Invest Dermatol. 1999;112:476–482. doi: 10.1046/j.1523-1747.1999.00550.x. [DOI] [PubMed] [Google Scholar]
- 37.Dieli F, Sireci G, Scire E, Salerno A, Bellavia A. Immunology. 1999;98:71–79. doi: 10.1046/j.1365-2567.1999.00844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noben-Trauth N, Kropf P, Muller I. Science. 1996;271:987–990. doi: 10.1126/science.271.5251.987. [DOI] [PubMed] [Google Scholar]
- 39.Mencacci A, Del Sero G, Cenci E, d'Ostiani C F, Bacci A, Montagnoli C, Kopf M, Romani L. J Exp Med. 1998;187:307–317. doi: 10.1084/jem.187.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]