Abstract
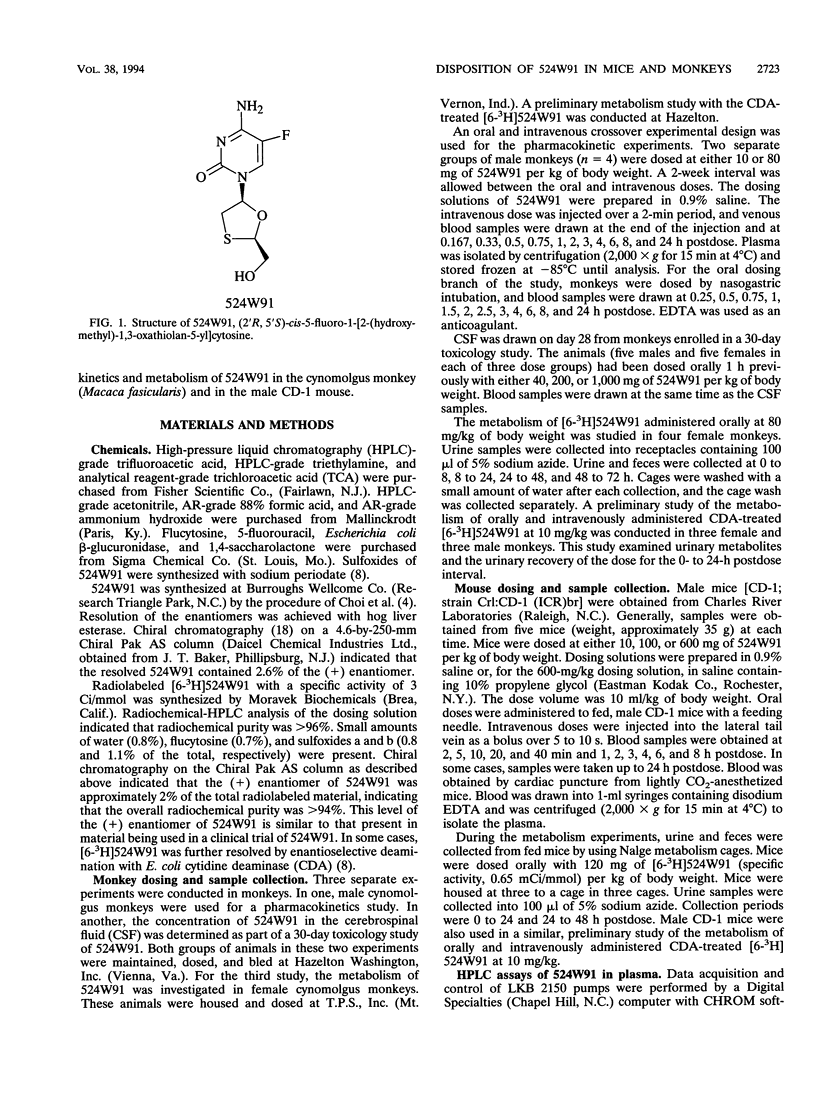
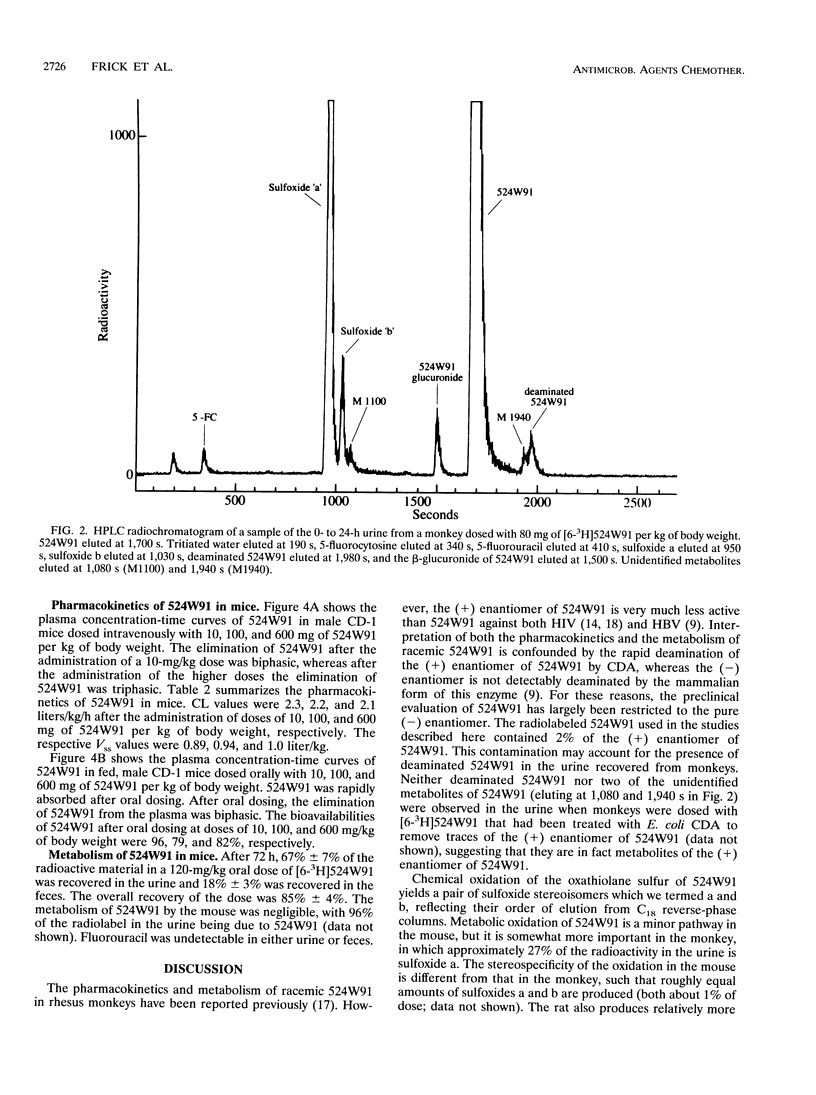
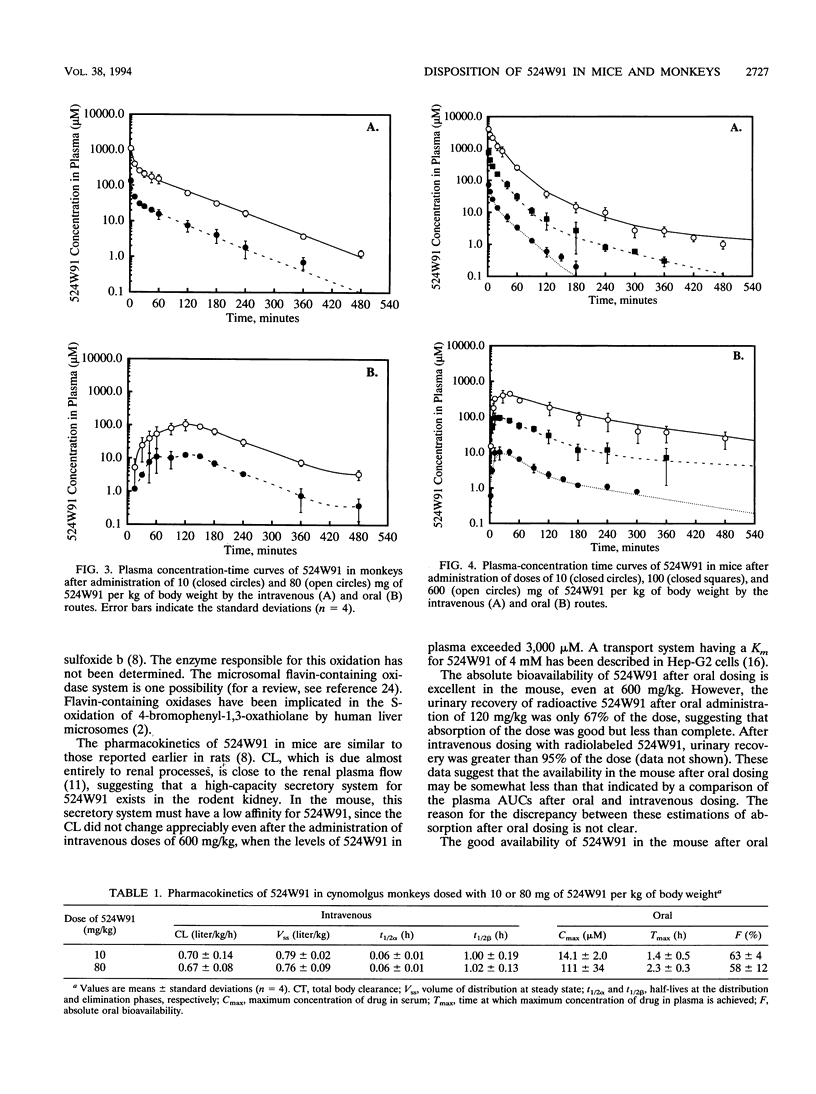
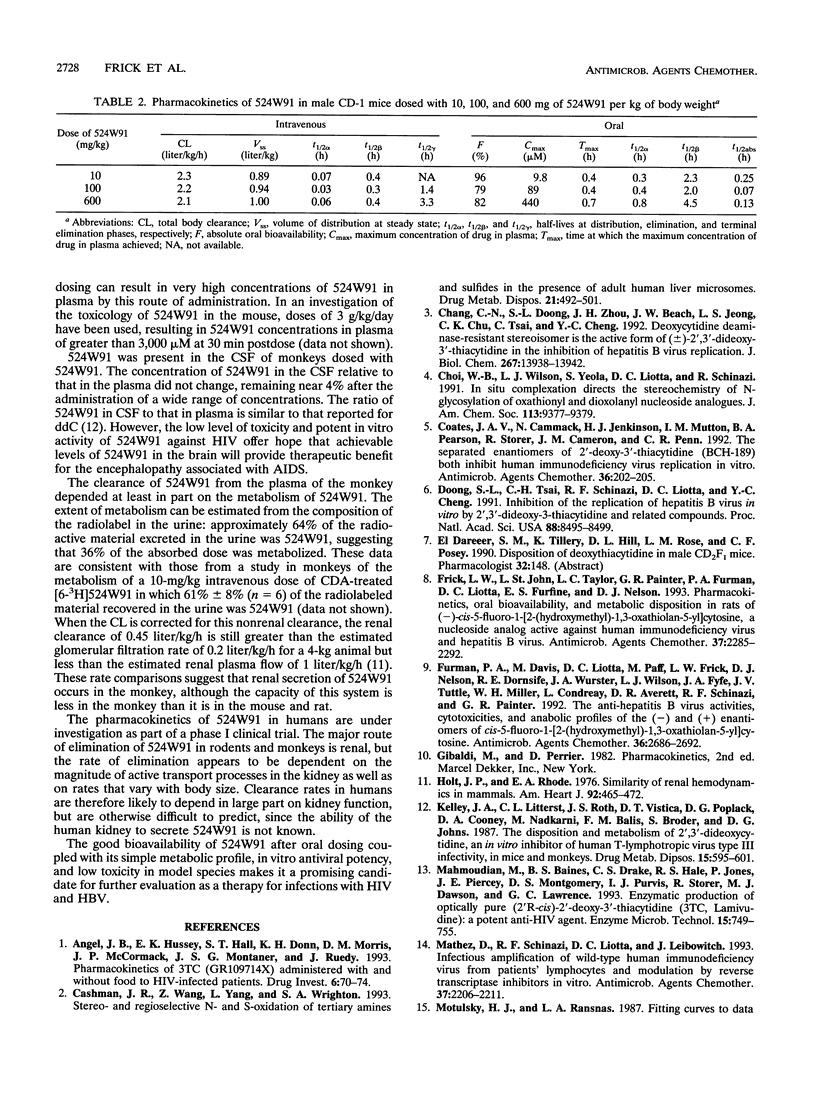
(2'R,5'S-)-cis-5-Fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl] cytosine (524W91) is a nucleoside analog with potent anti-human immunodeficiency virus and anti-human hepatitis B virus activities in vitro. The pharmacokinetics and bioavailability of 524W91 after oral dosing were studied in mice dosed with 10, 100, and 600 mg of 524W91 per kg of body weight by the oral and intravenous routes. Cynomolgus monkeys were dosed with 10 and 80 mg of 524W91 per kg. In both species, the clearance of 524W91 was rapid, via the kidney, and was independent of dose. In monkeys, the total body clearance of 10 mg of 524W91 per kg was 0.7 +/- 0.1 liter/h/kg, and the volume of distribution at steady state was 0.8 +/- 0.02 liter/kg. The terminal elimination half-life was 1.0 +/- 0.2 h. The absolute bioavailability after oral dosing was 63% +/- 4% at 10 mg/kg. Concentrations of 524W91 in the cerebrospinal fluid were 4% +/- 0.7% of the corresponding levels in plasma. In mice, the total clearance of 10 mg of 524W91 per kg was 2.3 liters/kg/h, and the volume of distribution at steady state was 0.9 liter/kg. Absolute bioavailability in mice after oral dosing was 96% at a dose of 10 mg/kg. The metabolism of orally administered [6-3H]524W91 was studied in cynomolgus monkeys at a dose of 80 mg/kg and in mice at a dose of 120 mg/kg. Monkeys excreted 41% +/- 6% of the radioactive dose in the 0- to 72-h urine, 33% +/- 10% in the feces, and 10% +/- 7% in the cage wash. Unchanged 524W91 was 64% of the total radiolabeled drug recovered in the urine. The glucuronide was a minor urinary metabolite. 5-Fluorouracil was not detected (less than 0.02% of the dose). Mice dosed orally with 120 mg of [6-3H]524W91 per kg excreted 67% +/- 7% of the radiolable in the )- to 48-h urine. Small amounts of the 3' -sulfoxide and glucuronide metabolites were observed in the urine, but 5-fluorouracil was not detected. Good bioavailability after oral dosing and resistance to metabolism recommend 524W91 for further preclinical evaluation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cashman J. R., Yang Z., Yang L., Wrighton S. A. Stereo- and regioselective N- and S-oxidation of tertiary amines and sulfides in the presence of adult human liver microsomes. Drug Metab Dispos. 1993 May-Jun;21(3):492–501. [PubMed] [Google Scholar]
- Chang C. N., Doong S. L., Zhou J. H., Beach J. W., Jeong L. S., Chu C. K., Tsai C. H., Cheng Y. C., Liotta D., Schinazi R. Deoxycytidine deaminase-resistant stereoisomer is the active form of (+/-)-2',3'-dideoxy-3'-thiacytidine in the inhibition of hepatitis B virus replication. J Biol Chem. 1992 Jul 15;267(20):13938–13942. [PubMed] [Google Scholar]
- Coates J. A., Cammack N., Jenkinson H. J., Mutton I. M., Pearson B. A., Storer R., Cameron J. M., Penn C. R. The separated enantiomers of 2'-deoxy-3'-thiacytidine (BCH 189) both inhibit human immunodeficiency virus replication in vitro. Antimicrob Agents Chemother. 1992 Jan;36(1):202–205. doi: 10.1128/aac.36.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong S. L., Tsai C. H., Schinazi R. F., Liotta D. C., Cheng Y. C. Inhibition of the replication of hepatitis B virus in vitro by 2',3'-dideoxy-3'-thiacytidine and related analogues. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8495–8499. doi: 10.1073/pnas.88.19.8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick L. W., St John L., Taylor L. C., Painter G. R., Furman P. A., Liotta D. C., Furfine E. S., Nelson D. J. Pharmacokinetics, oral bioavailability, and metabolic disposition in rats of (-)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl] cytosine, a nucleoside analog active against human immunodeficiency virus and hepatitis B virus. Antimicrob Agents Chemother. 1993 Nov;37(11):2285–2292. doi: 10.1128/aac.37.11.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., Davis M., Liotta D. C., Paff M., Frick L. W., Nelson D. J., Dornsife R. E., Wurster J. A., Wilson L. J., Fyfe J. A. The anti-hepatitis B virus activities, cytotoxicities, and anabolic profiles of the (-) and (+) enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992 Dec;36(12):2686–2692. doi: 10.1128/aac.36.12.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. P., Rhode E. A. Similarity of renal glomerular hemodynamics in mammals. Am Heart J. 1976 Oct;92(4):465–472. doi: 10.1016/s0002-8703(76)80046-4. [DOI] [PubMed] [Google Scholar]
- Kelley J. A., Litterst C. L., Roth J. S., Vistica D. T., Poplack D. G., Cooney D. A., Nadkarni M., Balis F. M., Broder S., Johns D. G. The disposition and metabolism of 2',3'-dideoxycytidine, an in vitro inhibitor of human T-lymphotrophic virus type III infectivity, in mice and monkeys. Drug Metab Dispos. 1987 Sep-Oct;15(5):595–601. [PubMed] [Google Scholar]
- Mahmoudian M., Baines B. S., Drake C. S., Hale R. S., Jones P., Piercey J. E., Montgomery D. S., Purvis I. J., Storer R., Dawson M. J. Enzymatic production of optically pure (2'R-cis)-2'-deoxy-3'-thiacytidine (3TC, lamivudine): a potent anti-HIV agent. Enzyme Microb Technol. 1993 Sep;15(9):749–755. doi: 10.1016/0141-0229(93)90005-m. [DOI] [PubMed] [Google Scholar]
- Mathez D., Schinazi R. F., Liotta D. C., Leibowitch J. Infectious amplification of wild-type human immunodeficiency virus from patients' lymphocytes and modulation by reverse transcriptase inhibitors in vitro. Antimicrob Agents Chemother. 1993 Oct;37(10):2206–2211. doi: 10.1128/aac.37.10.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky H. J., Ransnas L. A. Fitting curves to data using nonlinear regression: a practical and nonmathematical review. FASEB J. 1987 Nov;1(5):365–374. [PubMed] [Google Scholar]
- Paff M. T., Averett D. R., Prus K. L., Miller W. H., Nelson D. J. Intracellular metabolism of (-)- and (+)-cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine in HepG2 derivative 2.2.15 (subclone P5A) cells. Antimicrob Agents Chemother. 1994 Jun;38(6):1230–1238. doi: 10.1128/aac.38.6.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Boudinot F. D., Ibrahim S. S., Manning C., McClure H. M., Liotta D. C. Pharmacokinetics and metabolism of racemic 2',3'-dideoxy-5-fluoro-3'-thiacytidine in rhesus monkeys. Antimicrob Agents Chemother. 1992 Nov;36(11):2432–2438. doi: 10.1128/aac.36.11.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., Chu C. K., Peck A., McMillan A., Mathis R., Cannon D., Jeong L. S., Beach J. W., Choi W. B., Yeola S. Activities of the four optical isomers of 2',3'-dideoxy-3'-thiacytidine (BCH-189) against human immunodeficiency virus type 1 in human lymphocytes. Antimicrob Agents Chemother. 1992 Mar;36(3):672–676. doi: 10.1128/aac.36.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinazi R. F., McMillan A., Cannon D., Mathis R., Lloyd R. M., Peck A., Sommadossi J. P., St Clair M., Wilson J., Furman P. A. Selective inhibition of human immunodeficiency viruses by racemates and enantiomers of cis-5-fluoro-1-[2-(hydroxymethyl)-1,3-oxathiolan-5-yl]cytosine. Antimicrob Agents Chemother. 1992 Nov;36(11):2423–2431. doi: 10.1128/aac.36.11.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommadossi J. P., Schinazi R. F., Chu C. K., Xie M. Y. Comparison of cytotoxicity of the (-)- and (+)-enantiomer of 2',3'-dideoxy-3'-thiacytidine in normal human bone marrow progenitor cells. Biochem Pharmacol. 1992 Nov 17;44(10):1921–1925. doi: 10.1016/0006-2952(92)90093-x. [DOI] [PubMed] [Google Scholar]
- Soudeyns H., Yao X. I., Gao Q., Belleau B., Kraus J. L., Nguyen-Ba N., Spira B., Wainberg M. A. Anti-human immunodeficiency virus type 1 activity and in vitro toxicity of 2'-deoxy-3'-thiacytidine (BCH-189), a novel heterocyclic nucleoside analog. Antimicrob Agents Chemother. 1991 Jul;35(7):1386–1390. doi: 10.1128/aac.35.7.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler D. M. The 1990 Bernard B. Brodie Award Lecture. Unique properties of the enzymes of detoxication. Drug Metab Dispos. 1991 Sep-Oct;19(5):847–852. [PubMed] [Google Scholar]
- van Leeuwen R., Lange J. M., Hussey E. K., Donn K. H., Hall S. T., Harker A. J., Jonker P., Danner S. A. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS. 1992 Dec;6(12):1471–1475. doi: 10.1097/00002030-199212000-00008. [DOI] [PubMed] [Google Scholar]



