Abstract
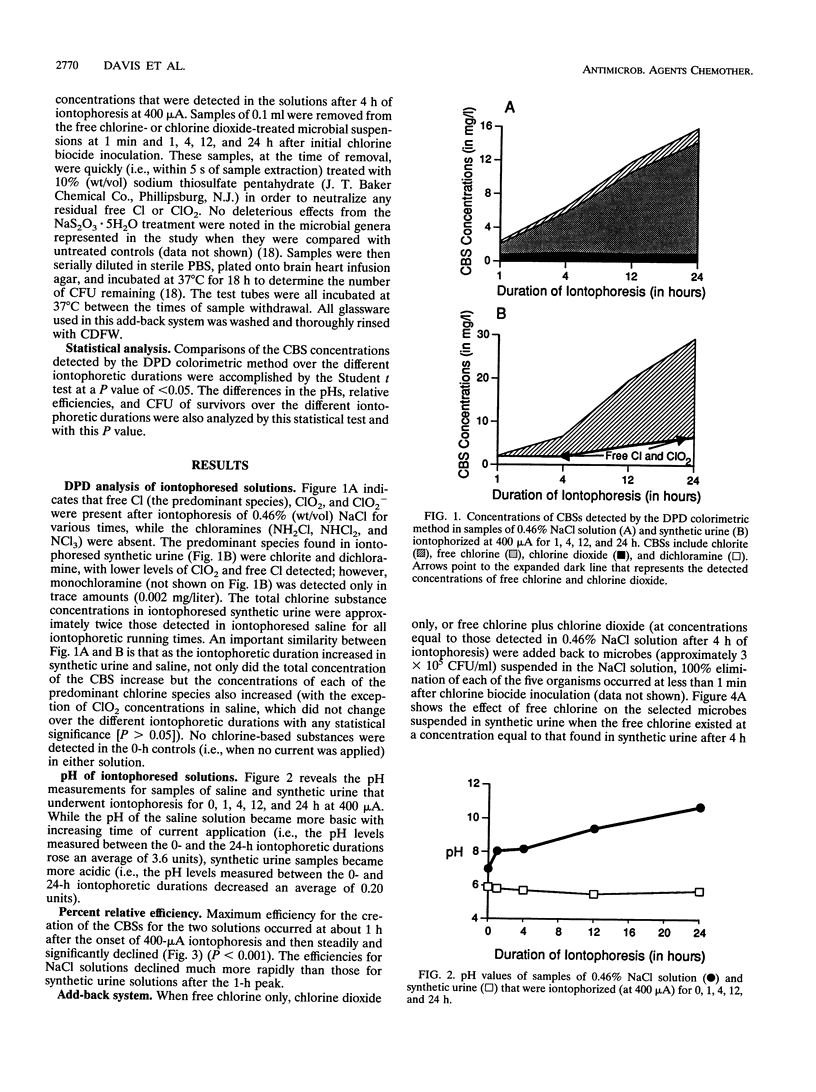
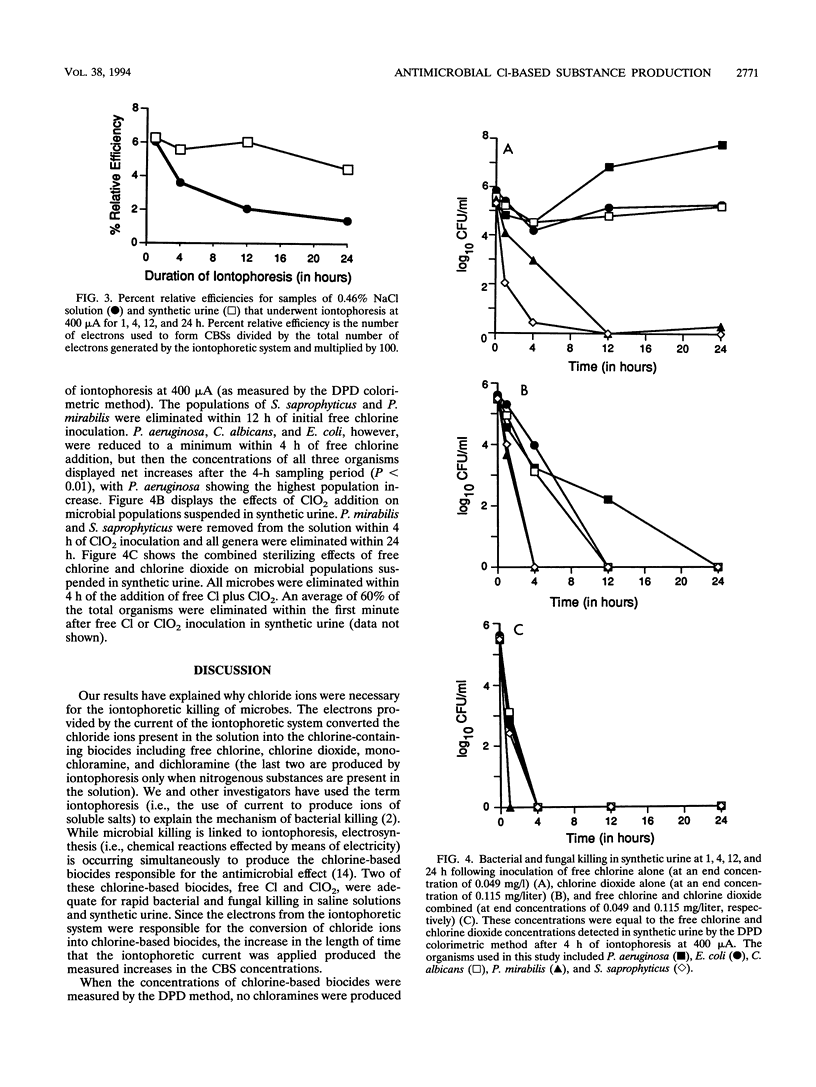
The dependence of microbial killing on chloride ions present in solutions undergoing iontophoresis is addressed. A 400-microA current was applied to vials containing synthetic urine or saline, and the production of chlorine-based substances (CBSs) was detected by the N,N-diethyl-p-phenylene diamine colorimetric method. It was found that as the time of current application increased, the total concentration of CBSs also increased. The iontophoretic current converted (through oxidation) chloride ions present in the solutions into CBSs such as free chlorine, chlorine dioxide, chlorite, monochloramine, and dichloramine (the last two were produced by iontophoresis only when nitrogenous substances were present in the solution). Two of the CBSs (free Cl and ClO2), when they were separately added back to microbial suspensions (approximately 3 x 10(5) CFU/ml) at the same concentrations at which they were detected in either 0.46% (wt/vol) NaCl solution or synthetic urine iontophoresed for 4 h at 400 microA, reduced or eliminated bacterial genera and a fungus. However, when free Cl and ClO2 were jointly added back to microbial suspensions, bacterial and fungal killing was synergistic and more rapid and complete than when these chlorine-based biocides were added separately. Therefore, iontophoresis of solutions containing chloride ions produces chlorine-based biocides that are responsible for the antimicrobial effect of iontophoresis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger T. J., Spadaro J. A., Bierman R., Chapin S. E., Becker R. O. Antifungal properties of electrically generated metallic ions. Antimicrob Agents Chemother. 1976 Nov;10(5):856–860. doi: 10.1128/aac.10.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzant J. L., Stephen R. L., Petelenz T. J., Jacobsen S. C. Painless cauterization of spider veins with the use of iontophoretic local anesthesia. J Am Acad Dermatol. 1988 Nov;19(5 Pt 1):869–875. doi: 10.1016/s0190-9622(88)70247-9. [DOI] [PubMed] [Google Scholar]
- Davis C. P., Anderson M. D., Hoskins S., Warren M. M. Electrode and bacterial survival with iontophoresis in synthetic urine. J Urol. 1992 May;147(5):1310–1313. doi: 10.1016/s0022-5347(17)37551-1. [DOI] [PubMed] [Google Scholar]
- Davis C. P., Arnett D., Warren M. M. Iontophoretic killing of Escherichia coli in static fluid and in a model catheter system. J Clin Microbiol. 1982 May;15(5):891–894. doi: 10.1128/jcm.15.5.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Wagel N., Anderson M. D., Warren M. M. Iontophoresis and chloride-containing compounds: parameters required for killing. J Urol. 1993 Oct;150(4):1172–1175. doi: 10.1016/s0022-5347(17)35717-8. [DOI] [PubMed] [Google Scholar]
- Davis C. P., Wagle N., Anderson M. D., Warren M. M. Bacterial and fungal killing by iontophoresis with long-lived electrodes. Antimicrob Agents Chemother. 1991 Oct;35(10):2131–2134. doi: 10.1128/aac.35.10.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Wagle N., Anderson M. D., Warren M. M. Iontophoresis generates an antimicrobial effect that remains after iontophoresis ceases. Antimicrob Agents Chemother. 1992 Nov;36(11):2552–2555. doi: 10.1128/aac.36.11.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Weinberg S., Anderson M. D., Rao G. M., Warren M. M. Effects of microamperage, medium, and bacterial concentration on iontophoretic killing of bacteria in fluid. Antimicrob Agents Chemother. 1989 Apr;33(4):442–447. doi: 10.1128/aac.33.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R. E. Effect of infections on hospital care. Ann Intern Med. 1978 Nov;89(5 Pt 2 Suppl):749–753. doi: 10.7326/0003-4819-89-5-749. [DOI] [PubMed] [Google Scholar]
- Griffith D. P., Musher D. M., Itin C. Urease. The primary cause of infection-induced urinary stones. Invest Urol. 1976 Mar;13(5):346–350. [PubMed] [Google Scholar]
- Harakeh S., Illescas A., Matin A. Inactivation of bacteria by Purogene. J Appl Bacteriol. 1988 May;64(5):459–463. doi: 10.1111/j.1365-2672.1988.tb05103.x. [DOI] [PubMed] [Google Scholar]
- Korich D. G., Mead J. R., Madore M. S., Sinclair N. A., Sterling C. R. Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl Environ Microbiol. 1990 May;56(5):1423–1428. doi: 10.1128/aem.56.5.1423-1428.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health Weekly Reports for DECEMBER 29, 1944. Public Health Rep. 1944 Dec 29;59(52):1661–1692. [PMC free article] [PubMed] [Google Scholar]
- Spector S. A., Tyndall M., Kelley E. Effects of acyclovir combined with other antiviral agents on human cytomegalovirus. Am J Med. 1982 Jul 20;73(1A):36–39. doi: 10.1016/0002-9343(82)90060-2. [DOI] [PubMed] [Google Scholar]



