Abstract
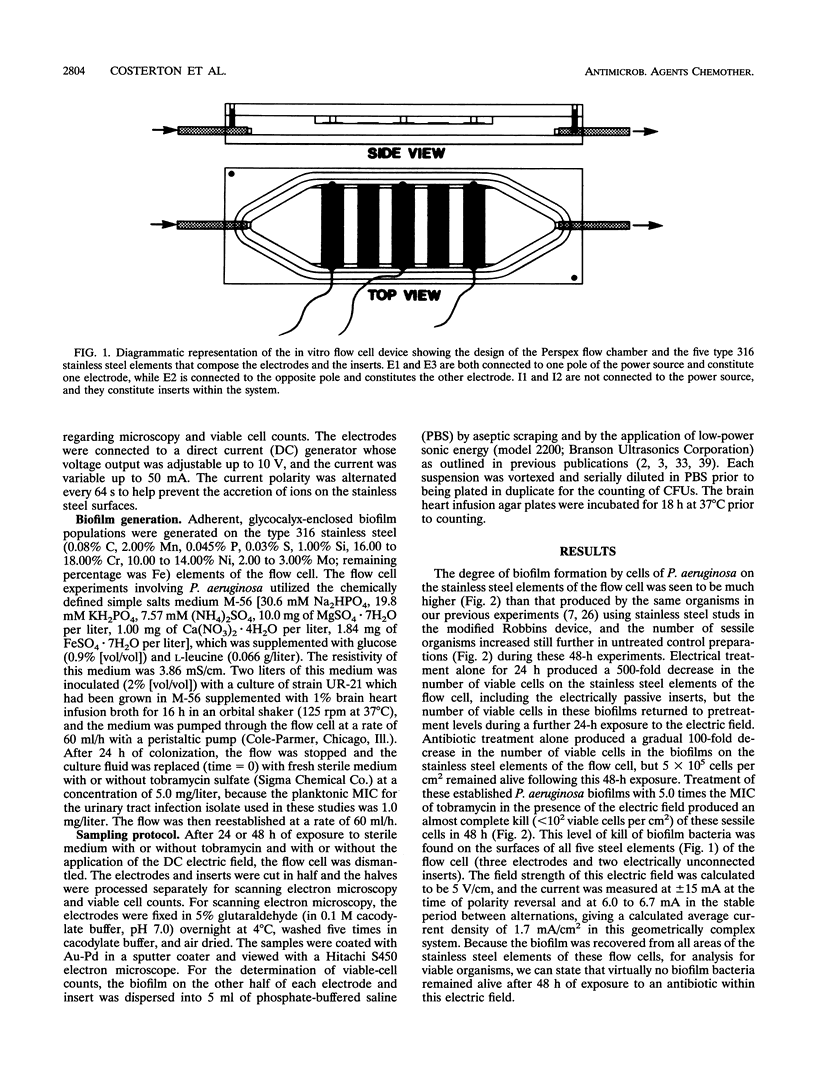
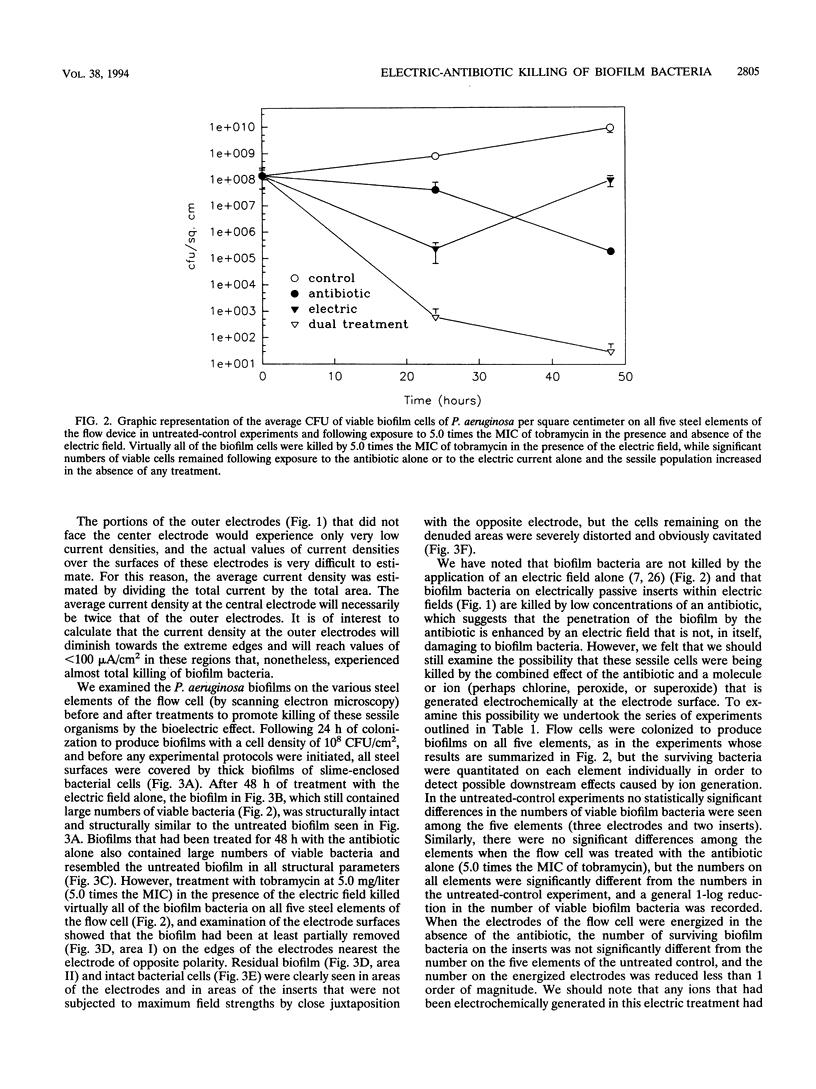
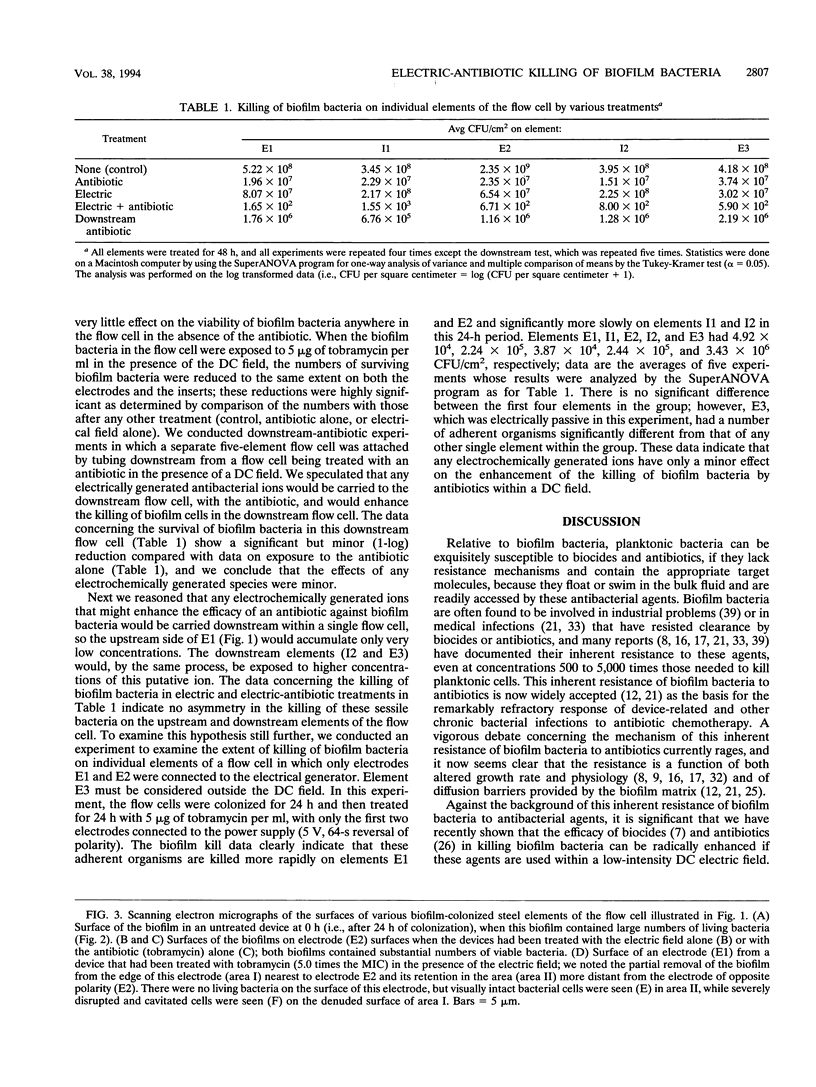
The bioelectric effect, in which electric fields are used to enhance the efficacy of biocides and antibiotics in killing biofilm bacteria, has been shown to reduce the very high concentrations of these antibacterial agents needed to kill biofilm bacteria to levels very close to those needed to kill planktonic (floating) bacteria of the same species. In this report, we show that biofilm bacteria are readily killed by an antibiotic on all areas of the active electrodes and on the surfaces of conductive elements that lie within the electric field but do not themselves function as electrodes. Considerations of electrode geometry indicate that very low (< 100 microA/cm2) current densities may be effective in this electrical enhancement of antibiotic efficacy against biofilm bacteria, and flow experiments indicate that this bioelectric effect does not appear to depend entirely on the possible local electrochemical generation of antibacterial molecules or ions. These data are expected to facilitate the use of the bioelectric effect in the prevention and treatment of device-related bacterial infections that are caused by bacteria that grow in biofilms and thereby frustrate antibiotic chemotherapy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amory D. E., Dufey J. E. Model for the electrolytic environment and electrostatic properties of biomembranes. J Bioenerg Biomembr. 1985 Jun;17(3):151–174. doi: 10.1007/BF00751059. [DOI] [PubMed] [Google Scholar]
- Anwar H., Costerton J. W. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1990 Sep;34(9):1666–1671. doi: 10.1128/aac.34.9.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwar H., Dasgupta M. K., Costerton J. W. Testing the susceptibility of bacteria in biofilms to antibacterial agents. Antimicrob Agents Chemother. 1990 Nov;34(11):2043–2046. doi: 10.1128/aac.34.11.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belehradek J., Jr, Orlowski S., Ramirez L. H., Pron G., Poddevin B., Mir L. M. Electropermeabilization of cells in tissues assessed by the qualitative and quantitative electroloading of bleomycin. Biochim Biophys Acta. 1994 Feb 23;1190(1):155–163. doi: 10.1016/0005-2736(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Berger T. J., Spadaro J. A., Chapin S. E., Becker R. O. Electrically generated silver ions: quantitative effects on bacterial and mammalian cells. Antimicrob Agents Chemother. 1976 Feb;9(2):357–358. doi: 10.1128/aac.9.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blenkinsopp S. A., Khoury A. E., Costerton J. W. Electrical enhancement of biocide efficacy against Pseudomonas aeruginosa biofilms. Appl Environ Microbiol. 1992 Nov;58(11):3770–3773. doi: 10.1128/aem.58.11.3770-3773.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. R., Allison D. G., Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? J Antimicrob Chemother. 1988 Dec;22(6):777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- Brown M. R., Collier P. J., Gilbert P. Influence of growth rate on susceptibility to antimicrobial agents: modification of the cell envelope and batch and continuous culture studies. Antimicrob Agents Chemother. 1990 Sep;34(9):1623–1628. doi: 10.1128/aac.34.9.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cevc G. Membrane electrostatics. Biochim Biophys Acta. 1990 Oct 8;1031(3):311–382. doi: 10.1016/0304-4157(90)90015-5. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Cheng K. J., Geesey G. G., Ladd T. I., Nickel J. C., Dasgupta M., Marrie T. J. Bacterial biofilms in nature and disease. Annu Rev Microbiol. 1987;41:435–464. doi: 10.1146/annurev.mi.41.100187.002251. [DOI] [PubMed] [Google Scholar]
- Davis C. P., Wagle N., Anderson M. D., Warren M. M. Bacterial and fungal killing by iontophoresis with long-lived electrodes. Antimicrob Agents Chemother. 1991 Oct;35(10):2131–2134. doi: 10.1128/aac.35.10.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Wagle N., Anderson M. D., Warren M. M. Iontophoresis generates an antimicrobial effect that remains after iontophoresis ceases. Antimicrob Agents Chemother. 1992 Nov;36(11):2552–2555. doi: 10.1128/aac.36.11.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. P., Weinberg S., Anderson M. D., Rao G. M., Warren M. M. Effects of microamperage, medium, and bacterial concentration on iontophoretic killing of bacteria in fluid. Antimicrob Agents Chemother. 1989 Apr;33(4):442–447. doi: 10.1128/aac.33.4.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng R. H., Padberg F. T., Smith S. M., Tan E. N., Cherubin C. E. Bactericidal effects of antibiotics on slowly growing and nongrowing bacteria. Antimicrob Agents Chemother. 1991 Sep;35(9):1824–1828. doi: 10.1128/aac.35.9.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert P., Collier P. J., Brown M. R. Influence of growth rate on susceptibility to antimicrobial agents: biofilms, cell cycle, dormancy, and stringent response. Antimicrob Agents Chemother. 1990 Oct;34(10):1865–1868. doi: 10.1128/aac.34.10.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R. W., Leikin S. L., Chernomordik L. V., Pastushenko V. F., Sokirko A. I. Reversible electrical breakdown of lipid bilayers: formation and evolution of pores. Biochim Biophys Acta. 1988 May 24;940(2):275–287. doi: 10.1016/0005-2736(88)90202-7. [DOI] [PubMed] [Google Scholar]
- Goodman R., Bassett C. A., Henderson A. S. Pulsing electromagnetic fields induce cellular transcription. Science. 1983 Jun 17;220(4603):1283–1285. doi: 10.1126/science.6857248. [DOI] [PubMed] [Google Scholar]
- Gristina A. G., Dobbins J. J., Giammara B., Lewis J. C., DeVries W. C. Biomaterial-centered sepsis and the total artificial heart. Microbial adhesion vs tissue integration. JAMA. 1988 Feb 12;259(6):870–874. [PubMed] [Google Scholar]
- Hancock R. E. Alterations in outer membrane permeability. Annu Rev Microbiol. 1984;38:237–264. doi: 10.1146/annurev.mi.38.100184.001321. [DOI] [PubMed] [Google Scholar]
- Hoyle B. D., Alcantara J., Costerton J. W. Pseudomonas aeruginosa biofilm as a diffusion barrier to piperacillin. Antimicrob Agents Chemother. 1992 Sep;36(9):2054–2056. doi: 10.1128/aac.36.9.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury A. E., Lam K., Ellis B., Costerton J. W. Prevention and control of bacterial infections associated with medical devices. ASAIO J. 1992 Jul-Sep;38(3):M174–M178. doi: 10.1097/00002480-199207000-00013. [DOI] [PubMed] [Google Scholar]
- McLaughlin S. The electrostatic properties of membranes. Annu Rev Biophys Biophys Chem. 1989;18:113–136. doi: 10.1146/annurev.bb.18.060189.000553. [DOI] [PubMed] [Google Scholar]
- Mouneimne Y., Tosi P. F., Barhoumi R., Nicolau C. Electroinsertion of xeno proteins in red blood cell membranes yields a long lived protein carrier in circulation. Biochim Biophys Acta. 1991 Jul 1;1066(1):83–89. doi: 10.1016/0005-2736(91)90254-6. [DOI] [PubMed] [Google Scholar]
- Nichols W. W., Evans M. J., Slack M. P., Walmsley H. L. The penetration of antibiotics into aggregates of mucoid and non-mucoid Pseudomonas aeruginosa. J Gen Microbiol. 1989 May;135(5):1291–1303. doi: 10.1099/00221287-135-5-1291. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Ruseska I., Wright J. B., Costerton J. W. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985 Apr;27(4):619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajnicek A. M., McCaig C. D., Gow N. A. Electric fields induce curved growth of Enterobacter cloacae, Escherichia coli, and Bacillus subtilis cells: implications for mechanisms of galvanotropism and bacterial growth. J Bacteriol. 1994 Feb;176(3):702–713. doi: 10.1128/jb.176.3.702-713.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabelnikov A. G., Cymbalyuk E. S., Gongadze G., Borovyagin V. L. Escherichia coli membranes during electrotransformation: an electron microscopy study. Biochim Biophys Acta. 1991 Jul 1;1066(1):21–28. doi: 10.1016/0005-2736(91)90245-4. [DOI] [PubMed] [Google Scholar]
- Shi W., Lentz M. J., Adler J. Behavioral responses of Escherichia coli to changes in temperature caused by electric shock. J Bacteriol. 1993 Sep;175(18):5785–5790. doi: 10.1128/jb.175.18.5785-5790.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsong T. Y. Electrical modulation of membrane proteins: enforced conformational oscillations and biological energy and signal transductions. Annu Rev Biophys Biophys Chem. 1990;19:83–106. doi: 10.1146/annurev.bb.19.060190.000503. [DOI] [PubMed] [Google Scholar]





