Abstract
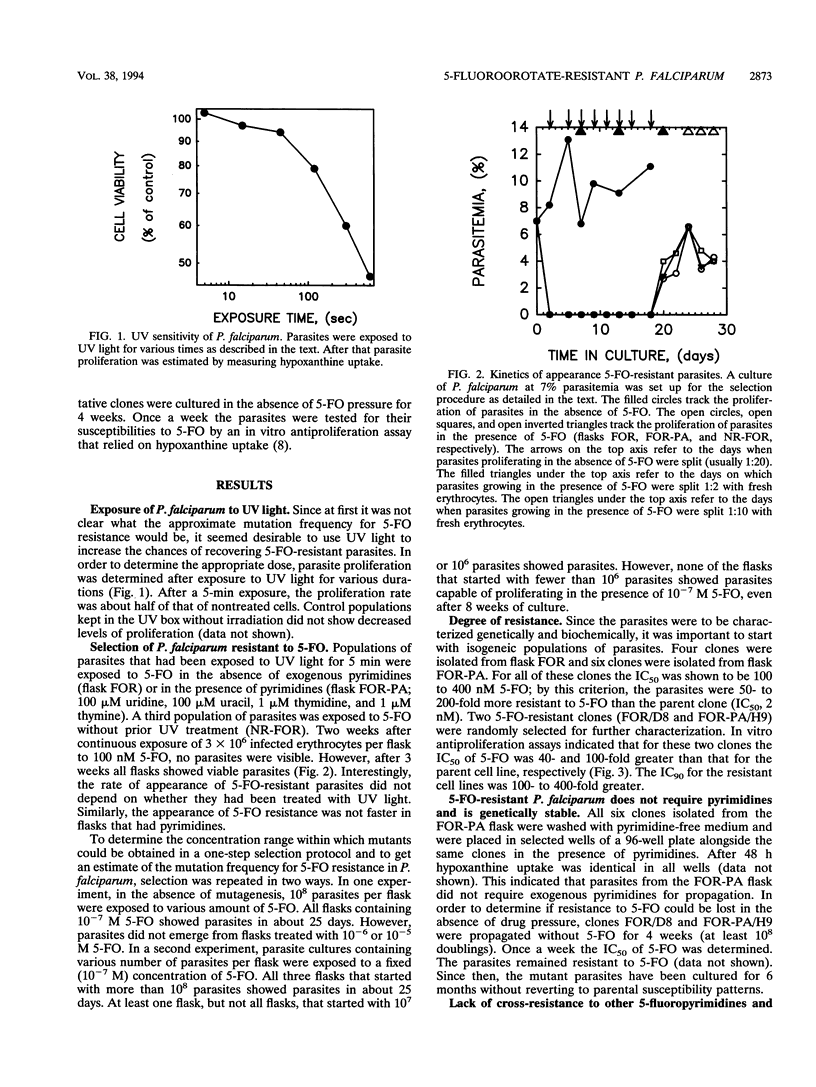
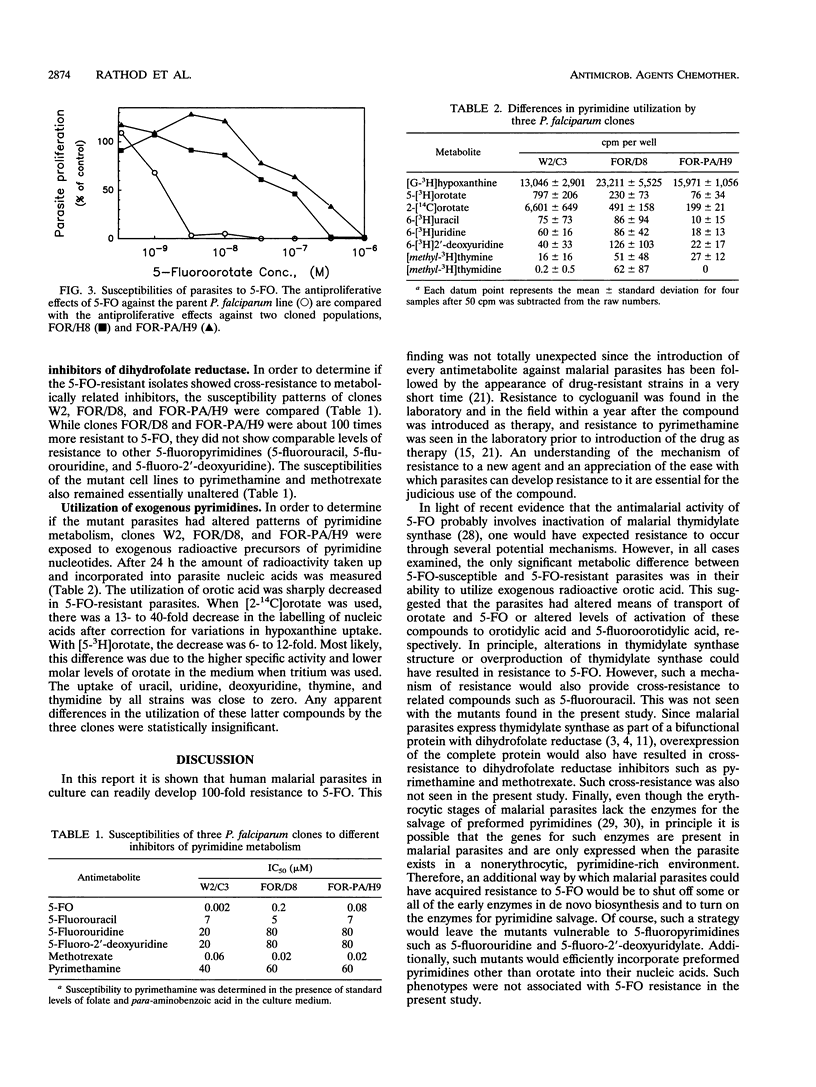
Previous studies have shown that 100 nM 5-fluoroorotate (5-FO) is sufficient to block the in vitro proliferation of Plasmodium falciparum without causing toxicity to mammalian cells. In anticipation of potential drug resistance, a study was undertaken to identify P. falciparum cells that would proliferate in the presence of 5-FO. About 3 x 10(6) UV-irradiated as well as nonirradiated parasites were subjected to a one-step selection with 100 nM 5-FO both in the absence and in the presence of preformed pyrimidines (uracil, uridine, thymine, and thymidine). The P. falciparum cells that emerged after 3 weeks were cloned, and the 90% inhibitory concentration of 5-FO for the cloned cells was found to be 100- to 400-fold greater than that for the parent cell line. Two clones that were further characterized retained resistance to 5-FO even after prolonged propagation in culture without drug pressure. Since the mutants were not cross-resistant to 5-fluorouracil or to dihydrofolate reductase inhibitors, it was unlikely that alteration of thymidylate synthase or overproduction of the bifunctional dihydrofolate reductase-thymidylate synthase was responsible for 5-FO resistance. Similarly, resistance was not due to the expression of a pyrimidine salvage pathway since the cells were not pyrimidine auxotrophs, they did not show increased utilization of pyrimidine nucleosides, and they did not show increased susceptibility to 5-fluoropyrimidine nucleosides. When the selection experiments were repeated, without mutagenesis, in the presence of 10(-7) M 5-FO with fewer than 10(6) parasites or in the presence of more than 10(-7) M 5-FO with more than 10(8) parasites, viable mutants could not be recovered from the cultures. The implications of these findings for the in vivo use of 5-FO for malaria chemotherapy are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banyal H. S., Inselburg J. Plasmodium falciparum: induction, selection, and characterization of pyrimethamine-resistant mutants. Exp Parasitol. 1986 Aug;62(1):61–70. doi: 10.1016/0014-4894(86)90008-1. [DOI] [PubMed] [Google Scholar]
- Beverley S. M., Coderre J. A., Santi D. V., Schimke R. T. Unstable DNA amplifications in methotrexate-resistant Leishmania consist of extrachromosomal circles which relocalize during stabilization. Cell. 1984 Sep;38(2):431–439. doi: 10.1016/0092-8674(84)90498-7. [DOI] [PubMed] [Google Scholar]
- Bzik D. J., Li W. B., Horii T., Inselburg J. Molecular cloning and sequence analysis of the Plasmodium falciparum dihydrofolate reductase-thymidylate synthase gene. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8360–8364. doi: 10.1073/pnas.84.23.8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. X., Zolg J. W. Purification of the bifunctional thymidylate synthase-dihydrofolate reductase complex from the human malaria parasite Plasmodium falciparum. Mol Pharmacol. 1987 Dec;32(6):723–730. [PubMed] [Google Scholar]
- Coderre J. A., Beverley S. M., Schimke R. T., Santi D. V. Overproduction of a bifunctional thymidylate synthetase-dihydrofolate reductase and DNA amplification in methotrexate-resistant Leishmania tropica. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2132–2136. doi: 10.1073/pnas.80.8.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coons T., Hanson S., Bitonti A. J., McCann P. P., Ullman B. Alpha-difluoromethylornithine resistance in Leishmania donovani is associated with increased ornithine decarboxylase activity. Mol Biochem Parasitol. 1990 Feb;39(1):77–89. doi: 10.1016/0166-6851(90)90010-j. [DOI] [PubMed] [Google Scholar]
- Cowman A. F., Morry M. J., Biggs B. A., Cross G. A., Foote S. J. Amino acid changes linked to pyrimethamine resistance in the dihydrofolate reductase-thymidylate synthase gene of Plasmodium falciparum. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9109–9113. doi: 10.1073/pnas.85.23.9109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detke S., Katakura K., Chang K. P. DNA amplification in arsenite-resistant Leishmania. Exp Cell Res. 1989 Jan;180(1):161–170. doi: 10.1016/0014-4827(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Ellenberger T. E., Beverley S. M. Reductions in methotrexate and folate influx in methotrexate-resistant lines of Leishmania major are independent of R or H region amplification. J Biol Chem. 1987 Oct 5;262(28):13501–13506. [PubMed] [Google Scholar]
- Garrett C. E., Coderre J. A., Meek T. D., Garvey E. P., Claman D. M., Beverley S. M., Santi D. V. A bifunctional thymidylate synthetase-dihydrofolate reductase in protozoa. Mol Biochem Parasitol. 1984 Apr;11:257–265. doi: 10.1016/0166-6851(84)90070-7. [DOI] [PubMed] [Google Scholar]
- Gómez Z. M., Rathod P. K. Antimalarial activity of a combination of 5-fluoroorotate and uridine in mice. Antimicrob Agents Chemother. 1990 Jul;34(7):1371–1375. doi: 10.1128/aac.34.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson S., Beverley S. M., Wagner W., Ullman B. Unstable amplification of two extrachromosomal elements in alpha-difluoromethylornithine-resistant Leishmania donovani. Mol Cell Biol. 1992 Dec;12(12):5499–5507. doi: 10.1128/mcb.12.12.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J. D., Diggs C. L., Hines F. A., Desjardins R. E. Culture of human malaria parasites Plasmodium falciparum. Nature. 1976 Oct 28;263(5580):767–769. doi: 10.1038/263767a0. [DOI] [PubMed] [Google Scholar]
- Iovannisci D. M., Kaur K., Young L., Ullman B. Genetic analysis of nucleoside transport in Leishmania donovani. Mol Cell Biol. 1984 Jun;4(6):1013–1019. doi: 10.1128/mcb.4.6.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur K., Coons T., Emmett K., Ullman B. Methotrexate-resistant Leishmania donovani genetically deficient in the folate-methotrexate transporter. J Biol Chem. 1988 May 25;263(15):7020–7028. [PubMed] [Google Scholar]
- Oduola A. M., Weatherly N. F., Bowdre J. H., Desjardins R. E. Plasmodium falciparum: cloning by single-erythrocyte micromanipulation and heterogeneity in vitro. Exp Parasitol. 1988 Jun;66(1):86–95. doi: 10.1016/0014-4894(88)90053-7. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Di Santi S. M., Povoa M., Calvosa V. S., Do Rosario V. E., Wellems T. E. Prevalence of the dihydrofolate reductase Asn-108 mutation as the basis for pyrimethamine-resistant falciparum malaria in the Brazilian Amazon. Am J Trop Med Hyg. 1991 Oct;45(4):492–497. doi: 10.4269/ajtmh.1991.45.492. [DOI] [PubMed] [Google Scholar]
- Peterson D. S., Milhous W. K., Wellems T. E. Molecular basis of differential resistance to cycloguanil and pyrimethamine in Plasmodium falciparum malaria. Proc Natl Acad Sci U S A. 1990 Apr;87(8):3018–3022. doi: 10.1073/pnas.87.8.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson D. S., Walliker D., Wellems T. E. Evidence that a point mutation in dihydrofolate reductase-thymidylate synthase confers resistance to pyrimethamine in falciparum malaria. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9114–9118. doi: 10.1073/pnas.85.23.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. A., Wang C. C. A Trypanosoma brucei mutant resistant to alpha-difluoromethylornithine. Mol Biochem Parasitol. 1987 Jan 2;22(1):9–17. doi: 10.1016/0166-6851(87)90064-8. [DOI] [PubMed] [Google Scholar]
- Rathod P. K., Gomez Z. M. Plasmodium yoelii: oral delivery of 5-fluoroorotate to treat malaria in mice. Exp Parasitol. 1991 Nov;73(4):512–514. doi: 10.1016/0014-4894(91)90075-8. [DOI] [PubMed] [Google Scholar]
- Rathod P. K., Khatri A., Hubbert T., Milhous W. K. Selective activity of 5-fluoroorotic acid against Plasmodium falciparum in vitro. Antimicrob Agents Chemother. 1989 Jul;33(7):1090–1094. doi: 10.1128/aac.33.7.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathod P. K., Leffers N. P., Young R. D. Molecular targets of 5-fluoroorotate in the human malaria parasite, Plasmodium falciparum. Antimicrob Agents Chemother. 1992 Apr;36(4):704–711. doi: 10.1128/aac.36.4.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes P., Rathod P. K., Sanchez D. J., Mrema J. E., Rieckmann K. H., Heidrich H. G. Enzymes of purine and pyrimidine metabolism from the human malaria parasite, Plasmodium falciparum. Mol Biochem Parasitol. 1982 May;5(5):275–290. doi: 10.1016/0166-6851(82)90035-4. [DOI] [PubMed] [Google Scholar]
- Sherman I. W. Biochemistry of Plasmodium (malarial parasites). Microbiol Rev. 1979 Dec;43(4):453–495. doi: 10.1128/mr.43.4.453-495.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Young R. D., Rathod P. K. Clonal viability measurements on Plasmodium falciparum to assess in vitro schizonticidal activity of leupeptin, chloroquine, and 5-fluoroorotate. Antimicrob Agents Chemother. 1993 May;37(5):1102–1107. doi: 10.1128/aac.37.5.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolg J. W., Plitt J. R., Chen G. X., Palmer S. Point mutations in the dihydrofolate reductase-thymidylate synthase gene as the molecular basis for pyrimethamine resistance in Plasmodium falciparum. Mol Biochem Parasitol. 1989 Oct;36(3):253–262. doi: 10.1016/0166-6851(89)90173-4. [DOI] [PubMed] [Google Scholar]



