Abstract
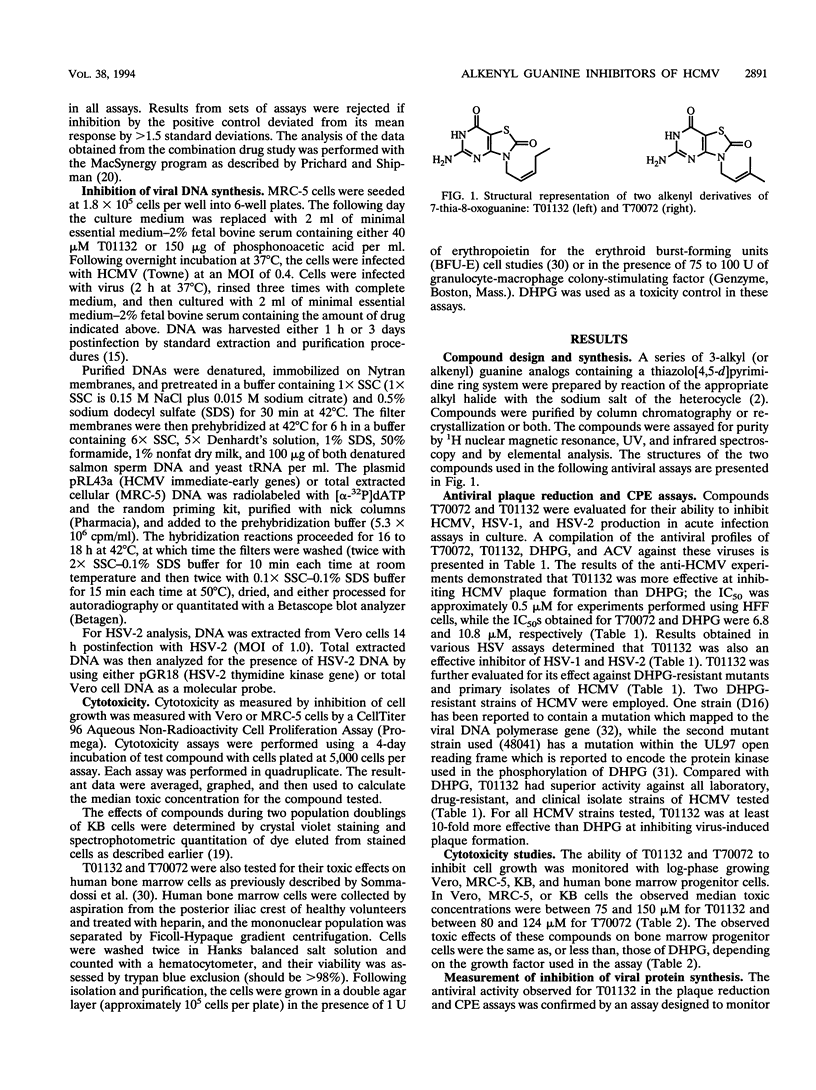
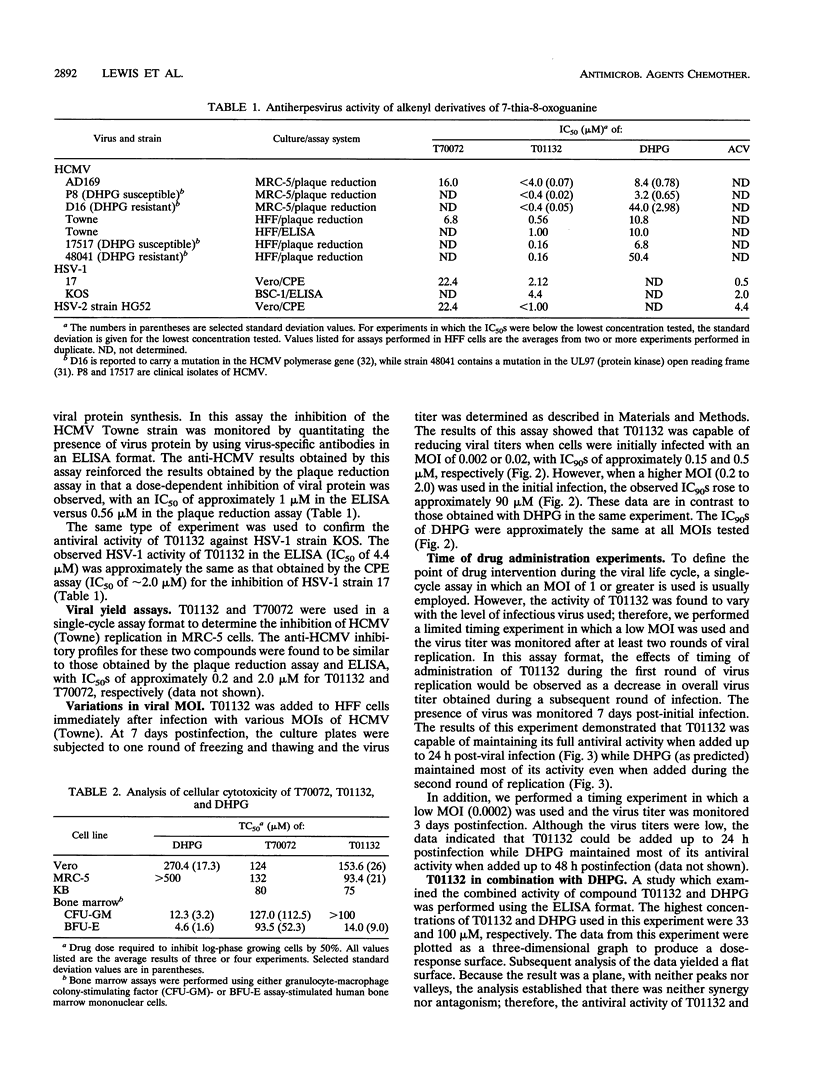
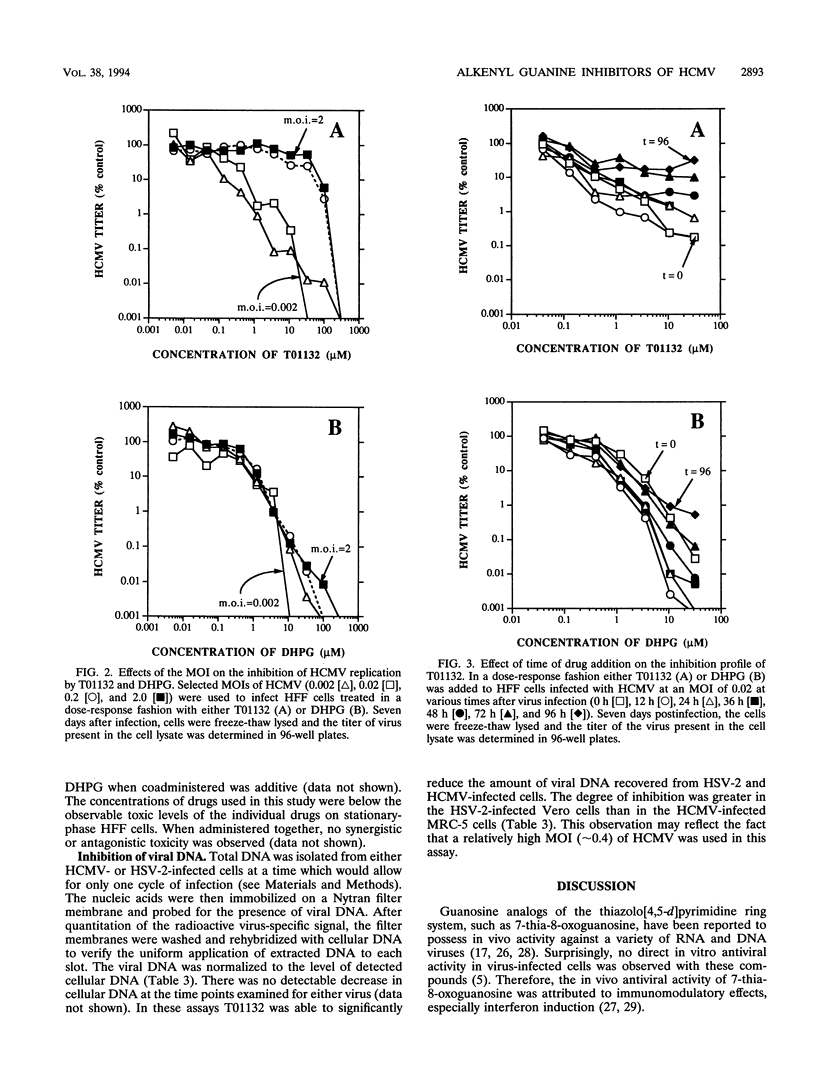
A series of alkyl and alkenyl guanine analogs containing a thiazolo[4,5-d]pyrimidine ring system were prepared by reaction of the appropriate alkyl halide with the sodium salt of the heterocycle. In preliminary antiviral efficacy evaluations against laboratory strains of both human cytomegalovirus (HCMV) and herpes simplex virus types 1 and 2, it was determined that two of the compounds (T70072 and T01132) were more active and less toxic in stationary-phase cell monolayers than were the other derivatives tested. T01132 and T70072, which have 2-pentenyl and 3-methyl-2-butenyl moieties attached to position 3 of the 5-aminothiazolo[4,5-d]pyrimidine-2,7-dione, respectively, were then more extensively evaluated for anti-HCMV activity. The concentrations of T01132 and T70072 required to inhibit HCMV by 50% in plaque reduction assays were approximately 0.5 and 6.8 microM, respectively. These two compounds inhibited the growth of KB, MRC-5, or Vero cells at concentrations of 75 to 150 microM, depending upon the cell line. In bone marrow progenitor cells T01132 was slightly less toxic than ganciclovir (DHPG). The 50% inhibitory concentrations of T01132 against clinical isolates and DHPG-resistant strains of HCMV were approximately the same as those obtained for laboratory strains of HCMV (approximately 0.5 microM). When tested in combination with DHPG, the resultant antiviral activity was determined to be additive but not synergistic. Experiments performed using variations of the viral multiplicity of infection (MOI) demonstrated that T01132 was more active than DHPG at a low MOI (0.002 or 0.02). However, when a higher MOI (0.2 or 2.0) was used, DHPG was more efficacious than T01132. In experiments in which drug was added at various times post-viral infection, T01132 was most effective when added within the first 24 h post-HCMV infection while DHPG was able to protect cells in this assay system when added up to 48 h postinfection, indicating that T01132 is exerting its antiviral effect on events leading up to and possibly including viral DNA synthesis. The data presented in this report suggest that the antiviral activity of alkenyl-substituted thiazolopyrimidine derivatives may represent a mechanism of action against herpesviruses alternative to that of classical nucleoside analogs such as acyclovir or DHPG.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Meurer L. C., Cantone C. L., Field A. K., Hannah J., Karkas J. D., Liou R., Patel G. F., Perry H. C., Wagner A. F. Synthesis and antiherpetic activity of (+/-)-9-[[(Z)-2-(hydroxymethyl)cyclopropyl]methyl]guanine and related compounds. J Med Chem. 1988 Dec;31(12):2304–2315. doi: 10.1021/jm00120a010. [DOI] [PubMed] [Google Scholar]
- Baker J. A., Chatfield P. V. Synthesis of derivatives of thiazolo[4,5-d]pyrimidine. II. J Chem Soc Perkin 1. 1970;18:2478–2484. doi: 10.1039/j39700002478. [DOI] [PubMed] [Google Scholar]
- Balfour H. H., Jr Management of cytomegalovirus disease with antiviral drugs. Rev Infect Dis. 1990 Sep-Oct;12 (Suppl 7):S849–S860. doi: 10.1093/clinids/12.supplement_7.s849. [DOI] [PubMed] [Google Scholar]
- Barnard D. L., Huffman J. H., Sidwell R. W., Reist E. J. Selective inhibition of cytomegaloviruses by 9-(3'-ethylphosphono-1'-hydroxymethyl-1'-propyloxy-methyl)g uanine. Antiviral Res. 1993 Sep;22(1):77–89. doi: 10.1016/0166-3542(93)90086-x. [DOI] [PubMed] [Google Scholar]
- Bonnet P. A., Robins R. K. Modulation of leukocyte genetic expression by novel purine nucleoside analogues. A new approach to antitumor and antiviral agents. J Med Chem. 1993 Mar 19;36(6):635–653. doi: 10.1021/jm00058a001. [DOI] [PubMed] [Google Scholar]
- Borcherding D. R., Narayanan S., Hasobe M., McKee J. G., Keller B. T., Borchardt R. T. Potential inhibitors of S-adenosylmethionine-dependent methyltransferases. 11. Molecular dissections of neplanocin A as potential inhibitors of S-adenosylhomocysteine hydrolase. J Med Chem. 1988 Sep;31(9):1729–1738. doi: 10.1021/jm00117a011. [DOI] [PubMed] [Google Scholar]
- Chrisp P., Clissold S. P. Foscarnet. A review of its antiviral activity, pharmacokinetic properties and therapeutic use in immunocompromised patients with cytomegalovirus retinitis. Drugs. 1991 Jan;41(1):104–129. doi: 10.2165/00003495-199141010-00009. [DOI] [PubMed] [Google Scholar]
- Drew W. L., Miner R. C., Busch D. F., Follansbee S. E., Gullett J., Mehalko S. G., Gordon S. M., Owen W. F., Jr, Matthews T. R., Buhles W. C. Prevalence of resistance in patients receiving ganciclovir for serious cytomegalovirus infection. J Infect Dis. 1991 Apr;163(4):716–719. doi: 10.1093/infdis/163.4.716. [DOI] [PubMed] [Google Scholar]
- Henderly D. E., Freeman W. R., Causey D. M., Rao N. A. Cytomegalovirus retinitis and response to therapy with ganciclovir. Ophthalmology. 1987 Apr;94(4):425–434. doi: 10.1016/s0161-6420(87)33454-2. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A., Drew W. L., Feinberg J., O'Donnell J. J., Whitmore P. V., Miner R. D., Parenti D. Foscarnet therapy for ganciclovir-resistant cytomegalovirus retinitis in patients with AIDS. J Infect Dis. 1991 Jun;163(6):1348–1351. doi: 10.1093/infdis/163.6.1348. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A. Review of the toxicities of foscarnet. J Acquir Immune Defic Syndr. 1992;5 (Suppl 1):S11–S17. [PubMed] [Google Scholar]
- Kini G. D., Anderson J. D., Sanghvi Y. S., Lewis A. F., Smee D. F., Revankar G. R., Robins R. K., Cottam H. B. Synthesis and antiviral activity of certain guanosine analogues in the thiazolo[4,5-d]pyrimidine ring system. J Med Chem. 1991 Oct;34(10):3006–3010. doi: 10.1021/jm00114a008. [DOI] [PubMed] [Google Scholar]
- Meyers J. D. Prevention and treatment of cytomegalovirus infection. Annu Rev Med. 1991;42:179–187. doi: 10.1146/annurev.me.42.020191.001143. [DOI] [PubMed] [Google Scholar]
- Nagahara K., Anderson J. D., Kini G. D., Dalley N. K., Larson S. B., Smee D. F., Jin A., Sharma B. S., Jolley W. B., Robins R. K. Thiazolo[4,5-d]pyrimidine nucleosides. The synthesis of certain 3-beta-D-ribofuranosylthiazolo[4,5-d]pyrimidines as potential immunotherapeutic agents. J Med Chem. 1990 Jan;33(1):407–415. doi: 10.1021/jm00163a064. [DOI] [PubMed] [Google Scholar]
- Pizzorno M. C., O'Hare P., Sha L., LaFemina R. L., Hayward G. S. trans-activation and autoregulation of gene expression by the immediate-early region 2 gene products of human cytomegalovirus. J Virol. 1988 Apr;62(4):1167–1179. doi: 10.1128/jvi.62.4.1167-1179.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard M. N., Prichard L. E., Baguley W. A., Nassiri M. R., Shipman C., Jr Three-dimensional analysis of the synergistic cytotoxicity of ganciclovir and zidovudine. Antimicrob Agents Chemother. 1991 Jun;35(6):1060–1065. doi: 10.1128/aac.35.6.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard M. N., Shipman C., Jr A three-dimensional model to analyze drug-drug interactions. Antiviral Res. 1990 Oct-Nov;14(4-5):181–205. doi: 10.1016/0166-3542(90)90001-n. [DOI] [PubMed] [Google Scholar]
- Prichard M. N., Turk S. R., Coleman L. A., Engelhardt S. L., Shipman C., Jr, Drach J. C. A microtiter virus yield reduction assay for the evaluation of antiviral compounds against human cytomegalovirus and herpes simplex virus. J Virol Methods. 1990 Apr;28(1):101–106. doi: 10.1016/0166-0934(90)90091-s. [DOI] [PubMed] [Google Scholar]
- Reyes G. R., Jeang K. T., Hayward G. S. Transfection with the isolated herpes simplex virus thymidine kinase genes. I. Minimal size of the active fragments from HSV-1 and HSV-2. J Gen Virol. 1982 Oct;62(Pt 2):191–206. doi: 10.1099/0022-1317-62-2-191. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969 Jan;130(1):305–310. doi: 10.3181/00379727-130-33543. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr, Smith S. H., Carlson R. H., Drach J. C. Antiviral activity of arabinosyladenine and arabinosylhypoxanthine in herpes simplex virus-infected KB cells: selective inhibition of viral deoxyribonucleic acid synthesis in synchronized suspension cultures. Antimicrob Agents Chemother. 1976 Jan;9(1):120–127. doi: 10.1128/aac.9.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D. F., Alaghamandan H. A., Cottam H. B., Jolley W. B., Robins R. K. Antiviral activity of the novel immune modulator 7-thia-8-oxoguanosine. J Biol Response Mod. 1990 Feb;9(1):24–32. [PubMed] [Google Scholar]
- Smee D. F., Alaghamandan H. A., Cottam H. B., Sharma B. S., Jolley W. B., Robins R. K. Broad-spectrum in vivo antiviral activity of 7-thia-8-oxoguanosine, a novel immunopotentiating agent. Antimicrob Agents Chemother. 1989 Sep;33(9):1487–1492. doi: 10.1128/aac.33.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smee D. F., Alaghamandan H. A., Jin A., Sharma B. S., Jolley W. B. Roles of interferon and natural killer cells in the antiviral activity of 7-thia-8-oxoguanosine against Semliki Forest virus infections in mice. Antiviral Res. 1990 Feb;13(2):91–102. doi: 10.1016/0166-3542(90)90025-3. [DOI] [PubMed] [Google Scholar]
- Sommadossi J. P., Schinazi R. F., Chu C. K., Xie M. Y. Comparison of cytotoxicity of the (-)- and (+)-enantiomer of 2',3'-dideoxy-3'-thiacytidine in normal human bone marrow progenitor cells. Biochem Pharmacol. 1992 Nov 17;44(10):1921–1925. doi: 10.1016/0006-2952(92)90093-x. [DOI] [PubMed] [Google Scholar]
- Sullivan V., Talarico C. L., Stanat S. C., Davis M., Coen D. M., Biron K. K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992 Jul 9;358(6382):162–164. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- Tatarowicz W. A., Lurain N. S., Thompson K. D. A ganciclovir-resistant clinical isolate of human cytomegalovirus exhibiting cross-resistance to other DNA polymerase inhibitors. J Infect Dis. 1992 Oct;166(4):904–907. doi: 10.1093/infdis/166.4.904. [DOI] [PubMed] [Google Scholar]
- Turk S. R., Shipman C., Jr, Nassiri R., Genzlinger G., Krawczyk S. H., Townsend L. B., Drach J. C. Pyrrolo[2,3-d]pyrimidine nucleosides as inhibitors of human cytomegalovirus. Antimicrob Agents Chemother. 1987 Apr;31(4):544–550. doi: 10.1128/aac.31.4.544. [DOI] [PMC free article] [PubMed] [Google Scholar]


