Abstract
Estrogen regulates the amount of white adipose tissue (WAT) in females, but its role in males and whether WAT effects involve estrogen receptor-α (ERα) or ERβ were unclear. We analyzed the role of ERα in WAT and brown adipose tissue by comparing these tissues in wild-type (WT) and ERα-knockout (αERKO) male and female mice. Brown adipose tissue weight was similar in αERKO and WT males at all ages. Progressive increases in WAT were seen in αERKO males with advancing age. Epididymal, perirenal, and inguinal WAT weighed 139–185% more in αERKO than in WT males by 270–360 days of age. Epididymal and perirenal adipocyte size was increased 20% in αERKO males. Adipocyte number was 82–168% greater in fat pads of αERKO vs. WT males. Compared with WT, 90-day-old αERKO females had increases in fat pad weights (54–103%), adipocyte size, and number. Both αERKO males and females had insulin resistance and impaired glucose tolerance, similar to humans lacking ERα or aromatase. Energy intake was equal in WT and αERKO males, indicating that obesity was not induced by hyperphagia. In contrast, energy expenditure was reduced by 11% in αERKO compared with WT males, indicating that altered energy expenditure may be important for the observed obesity. In summary, ERα absence causes adipocyte hyperplasia and hypertrophy, insulin resistance, and glucose intolerance in both sexes. These results are evidence that estrogen/ERα signaling is critical in female and male WAT; obesity in αERKO males involves a mechanism of reduced energy expenditure rather than increased energy intake.
Obesity is a significant human health problem whose incidence is reaching epidemic proportions in some Western countries. For example, obesity in Americans has risen dramatically in the past 40 years, from 12.8% in 1962 to 22.5% in 1998, and 55% of the population is considered overweight (1). Obesity is associated with increased type II diabetes, heart disease, certain cancers, and other health problems, and obesity is estimated to be responsible for 300,000 deaths/year in the U.S. (2). Because of these human health concerns, there is intense interest in factors that regulate development and function of white adipose tissue (WAT). In addition, factors regulating WAT in food animals are important because of concerns over excess fat consumption in Western diets, which may contribute to adverse health effects.
Evidence from both humans and laboratory animals suggests that estrogen plays an important role in WAT regulation. Ovariectomy of rodents increases WAT, and estrogen replacement decreases WAT (3). Similarly, postmenopausal women have increased WAT, and estrogen therapy decreases WAT levels compared with untreated postmenopausal women (4).
Female WAT expresses the classical estrogen receptor, estrogen receptor-α (ERα), as well as the recently described ERβ (5–8). Although the relative role of ERα and ERβ and the mechanism by which estrogen regulates WAT are unclear, estrogen effects on glucose homeostasis in females may be involved (9). For example, glucose tolerance and insulin concentrations vary across the estrous cycle in rodents (10), probably because of changing estrogen and progesterone. Ovariectomy in rodents impairs glucose tolerance and alters glucose-induced insulin secretion, whereas estrogen replacement partially or completely prevents this (9). These animal studies are consistent with reported reversal of reduced insulin sensitivity in postmenopausal women by estrogen (4, 11). Additionally, humans lacking either ERα or aromatase have insulin resistance (12, 13).
Brown adipose tissue (BAT) is found in humans and other mammals and plays a critical role in postnatal thermogenesis in rodents. Most investigations into the role of estrogen in adipose regulation have focused on WAT, and the role of estradiol 17-β (E2) and ER in BAT development and function is unclear.
As in females, male adipose tissue expresses ER (6). ER also is expressed in other organs associated with satiety and feeding, such as hypothalamus and pituitary, in the male. Despite the role of estrogen in female WAT, it was unknown whether estrogen binding to ERα and/or ERβ normally plays any role in regulating male WAT.
The development of ERα knockout (αERKO) mice provided a powerful tool to examine the role of E2/ERα signaling in development and function of various organs and tissues (14). In this study, we used male and female αERKO mice to determine whether absence of ERα expression produces phenotypic changes in WAT and BAT in either sex. Our results indicate that ERα absence results in marked increases in WAT, but not BAT, insulin resistance and impaired glucose tolerance in both males and females, and altered energy expenditure in males. These data indicate that ERα is critical for regulating female WAT deposition and directly implicate the E2/ERα signaling pathway as a major regulatory factor for male WAT.
Materials and Methods
Animals.
Animal experiments were approved by the Laboratory Animal Care Advisory Committees of the universities of Illinois and Missouri and conducted in accordance with Institutional Animal Care and Use Committee guidelines. Animals were maintained under standard temperature and lighting, received Teklad 8604 Rodent Diet (University of Illinois) or Purina 5001 Rodent Chow (University of Missouri) and water ad libitum, and were housed in polycarbonate cages with hardwood fiber chips. Homozygous wild-type (WT) and αERKO mice were obtained by mating mice heterozygous for the ERα gene disruption (14). Parental heterozygote mice used to generate WT and αERKO mice used in this study were derived by backcrossing the C57BL6/129SV (14) to C57BL/6J for 7–10 generations. Pup genotypes were determined by multiplex PCR (14).
Quantitation and Analysis of WAT and BAT.
WT and αERKO males of 5, 30, 90, 180, and 270–360 days of age (n ≥ 5 for all age groups) and female WT and αERKO mice at 90 days of age (n ≥ 4 for both groups) were used for analysis of WAT and BAT. For 5-day-old males, epididymal WAT was removed and weighed. For older males, epididymal, perirenal, and inguinal WAT, BAT from the interscapular region, and the kidneys were removed and weighed. In females, parametrial, perirenal, and inguinal WAT, BAT, and kidneys were removed and weighed. WAT samples were used for determination of adipocyte number and size.
Determination of Adipocyte Number and Area.
One hundred-milligram samples of all three WAT depots from 90-day-old female and 180-day-old male WT and αERKO mice (n ≥ 4 for all groups) were used for determination of adipocyte number and area. To prepare a single-cell suspension, WAT was incubated with collagenase in Krebs buffer (15) and passed through cheesecloth, and methylene blue was added to the adipocyte slurry. Adipocytes in 5.5 μl of suspension were quantified by using a hemocytometer, and total adipocyte number per sample was calculated by using the appropriate dilution factor.
To determine adipocyte areas, stained suspensions were photographed by using a Spot digital camera interfaced to an Olympus dissecting microscope and a Power Macintosh G3 computer. The areas of 120 adipocytes from each fat pad were measured in captured images by using the public domain National Institutes of Health image program.
Blood Glucose Measurements.
Adult male and female WT and αERKO mice were fasted for approximately 17 h overnight, with full access to water. Tail blood was collected by vein nicks. Blood glucose was measured by using a Glucometer Elite (Bayer) blood glucose meter (16) before glucose challenge (2 mg/g body weight in saline, i.p.), then again at 30, 60, and 120 min after glucose administration.
Serum Insulin Levels.
Insulin levels were measured in adult male and female αERKO and WT mice after overnight fasting. Animals received i.p. injections of either glucose (2 mg/kg body weight in saline) or saline and were killed before or after the glucose/saline challenge. Trunk blood was collected, and serum insulin concentrations were measured by RIA (Linco Research Immunoassay, St. Charles, MO) using rat insulin as a standard. Interassay and intraassay coefficients of variation were 7.4% and 4.7%, respectively, and assay sensitivity was 0.02 ng/ml.
Energy Expenditure Studies.
Increased fat in αERKO mice could result from increased food intake and/or decreased energy expenditure. To directly address this, body weight and food consumption of five αERKO/WT male sibling pairs were recorded 3×/week from 2 to 11 months of age. Energy intake was obtained by multiplying food intake by the caloric value of the chow (3.93 kcal/g), and feed efficiency was expressed as the increase in body weight (g)/kcal eaten. Energy expenditure was measured in seven αERKO/WT male sibling pairs at 10–11 months of age by using an open-circuit indirect calorimetry system (17), which continuously monitors O2 and CO2 concentrations by using separate analyzers (Applied Electrochemistry, Sunnyvale, CA).
Statistics.
Glucose, body weight, and feed consumption data were analyzed by repeated measures ANOVA, followed by least-square means tests, using the SAS Institute (Cary, NC) statistical package. Because a nonlinear relationship between body weight and age was present, a quadratic function was fitted to this relationship, and the coefficient values were compared by paired Student's t tests. Adipocyte area was analyzed by nested ANOVA, and other parameters were analyzed by an unpaired or paired Student's t test, as appropriate. A P < 0.05 was considered significant.
Results
Adipose Weight in Males and Females.
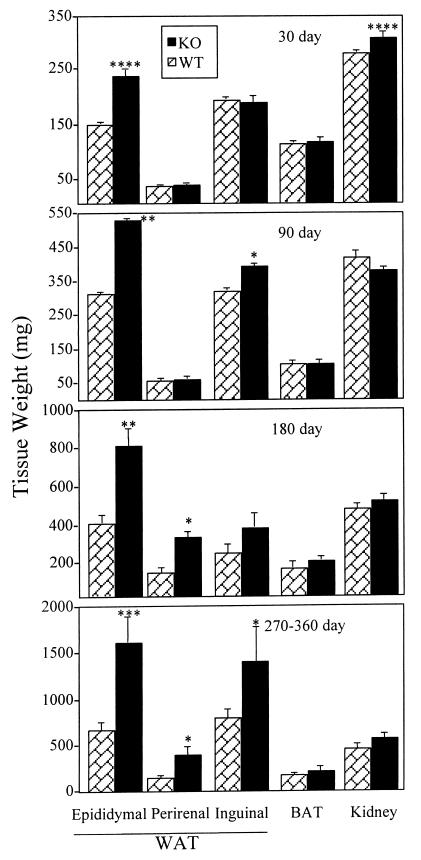
The role of E2/ERα signaling in adipose development and function was examined by using male and female αERKO and WT mice. Epididymal fat pad weights were similar in WT and αERKO males at 5 days postnatal (data not shown). In contrast, large increases in WAT were apparent in αERKO males at subsequent ages. At 30 days of age, epididymal fat in αERKO males weighed 64% more than WT, although there were no significant differences in perirenal and inguinal WAT (Fig. 1). At 90 days of age, both epididymal and inguinal fat pads were significantly larger in αERKO than WT males (70% and 20%, respectively). The increased WAT in αERKO males relative to WT continued to become more pronounced with advancing age. By 180 days, fat pad weights were 40–100% greater in αERKO than WT males. Finally, in αERKO males between 270 and 360 days of age, epididymal, perirenal, and inguinal fat pad weights were 139%, 185%, and 146% greater, respectively, than in WT. All increases at 180 and 270–360 days were significant except for that in the 180-day inguinal fat pad, which showed a trend toward an increase that did not reach significance (P = 0.10). At all ages, BAT weights were not different in αERKO and WT males (Fig. 1). Kidney weights in 90-, 180-, and 270- to 360-day αERKO males were not different from WT, suggesting that increased WAT in αERKO males is not simply a result of increased body growth.
Figure 1.
Weights of adipose and other tissues in WT and αERKO males at various ages. Data are expressed as mean ± SEM, and n ≥ 5 for both groups at all ages. Values significantly different from the age-matched WT controls are designated by asterisks above the column: ****, P < 0.001; **, P < 0.01; *, P < 0.05. Note different scales in each panel.
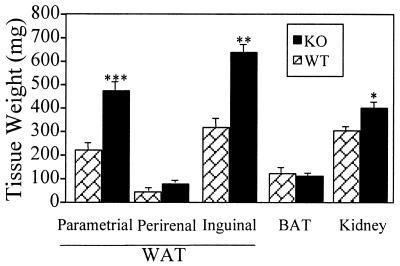
Compared with WT females, αERKO females showed marked increases in WAT (Fig. 2), consistent with those seen in αERKO males. At 90 days of age, parametrial, perirenal, and inguinal fat pads were 103%, 54%, and 96% greater, respectively in αERKO vs. WT females. As in males, BAT weights were not different between αERKO and WT females. Kidney and body weights were increased in 90-day-old αERKO females compared with WT, but as in older males, these increases were less than the WAT increases, indicating that increased WAT is not a nonspecific result of increased body growth.
Figure 2.
Weights of adipose and other tissues in 90-day-old WT and αERKO females. Data are expressed as mean ± SEM, and n ≥ 4 for both groups. Values significantly different from age-matched WTs are designated by asterisks above the column: ***, P < 0.005; **, P < 0.01; *, P < 0.05.
Adipocyte Number and Area.
Areas of epididymal and perirenal adipocytes from 180-day-old αERKO mice were significantly greater (18% and 19%, respectively) than those of WT (Table 1). Adipocyte number was 168% and 82% greater (P < 0.05) in epididymal and perirenal fat pads, respectively, from αERKO vs. WT males (Table 1), indicating that increased adipocyte volume in αERKO males was accompanied by increased adipocyte number. Inguinal adipocyte number and size showed a trend toward an increase that did not reach significance (P = 0.11 and 0.10, respectively). Significant increases in adipocyte number and size also were obtained in 90-day-old females (data not shown).
Table 1.
Adipocyte size and number in 180-day WT and αERKO males
| Fat pad | Genotype | Cell size, μm2 | Cell number, × 107 |
|---|---|---|---|
| Epididymal | WT | 1,339 ± 47 | 13.7 ± 5.1 |
| KO | 1,588 ± 56* | 36.7 ± 5.3* | |
| Perirenal | WT | 1,031 ± 32 | 7.4 ± 1.7 |
| KO | 1,221 ± 40* | 13.5 ± 1.9* | |
| Inguinal | WT | 1,204 ± 34 | 9.4 ± 3.5 |
| KO | 1,300 ± 49 | 26.7 ± 8.0 |
Data represent mean ± SEM (n ≥ 4).
A significant difference versus WT at P < 0.05.
Glucose Tolerance in WT vs. αERKO Mice.
Fasting blood glucose did not differ significantly between WT and αERKO males or females (Fig. 3). However, overall response to glucose challenge was different: by 30 min postchallenge, blood glucose levels in αERKO males rose from 93 ± 5 mg/dl at t = 0 to 358 ± 14 mg/dl, compared with a rise from 84 ± 8 mg/dl at t = 0 to 309 ± 16 mg/dl in WT males (Fig. 3A). This difference between WT and αERKO males persisted at 60 and 120 min after glucose injection. Similarly, glucose values were higher in αERKO compared with WT females at all times after glucose administration (Fig. 3B).
Figure 3.
Blood glucose concentrations in adult male (A) and female (B) WT (n = 8 and 13, respectively) and αERKO (n = 10 and 20, respectively) mice before and after a glucose challenge (2 mg/kg body weight); data are expressed as mean ± SEM. Blood glucose levels were not different before glucose injection in αERKO and WT mice of either sex, but both male and female αERKO mice had a more pronounced and sustained increase in blood glucose after the initial glucose injection; αERKO values in both sexes were significantly (P < 0.05) greater than WTs at other time points (30, 60, and 120 min).
Serum Insulin Levels.
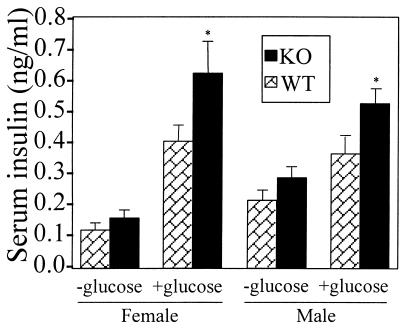
Glucose challenge caused a pronounced increase in insulin levels above basal levels in both αERKO and WT males and females (Fig. 4), and insulin levels at 30 min after glucose administration were increased by over 40% and 50% in αERKO males and females, respectively, compared with WT controls (P < 0.01).
Figure 4.
Serum insulin concentrations in adult male and female WT (n = 10 and 8, respectively) and αERKO (n = 10 and 8, respectively) mice 30 min after glucose challenge. Fasted mice were injected i.p. with saline or glucose (2 mg/kg body weight), then blood was collected 30 min later. Data are expressed as mean ± SEM. Basal serum insulin levels in mice not given glucose challenge were 30–35% higher in both αERKO males and females than WT (e.g., 0.11 ± 0.02 and 0.15 ± 0.02 ng/ml in WT and αERKO females, respectively) and showed a trend toward an increase compared with WT controls that did not reach statistical significance in either sex (P = 0.06 and 0.08, respectively). Insulin levels at 30 min after glucose challenge were increased over baseline in all mice and were more than 40% greater in glucose-challenged αERKO than WT males (P < 0.01). Similarly, insulin levels were 50% greater (P < 0.05) in αERKO vs. WT females after glucose challenge.
Body Weights, Food Consumption, and Energy Expenditure.
Body weights and food consumption in WT and αERKO males from 2–11 months of age are shown in Fig. 5. WT males are heavier at birth, but αERKO males catch up and their body weights are not significantly different (P > 0.05) at 2 months of age (Fig. 5A). Weights of αERKO males continued to increase relative to WT, and by 11 months of age αERKO males are 16% heavier (P < 0.05). Despite this, daily energy intake was not different in WT and αERKO males (14.9 ± 0.8 vs. 14.8 ± 0.8 kcal/day, respectively) during the period from 2 to 11 months of age (Fig. 5B), and average overall food consumption by the two groups during the entire period from 2–11 months was almost identical (3,394 ± 81 and 3,411 ± 74 kcal in WT and αERKO males, respectively). The greater increase in body weight in αERKO males despite unaltered food intake reflected a greater (P < 0.05) feed efficiency [increase in body weight (g)/kcal eaten] in the αERKO (0.0038 ± 0.0009) compared with WT (0.0024 ± 0.0005) males.
Figure 5.
Body weights (A) and food intake (B) of five male sibling pairs of WT (dashed lines) and αERKO (solid lines) mice from 2–11 months of age. A quadratic function was fit to the data for each group, which indicated significant differences in the linear (body weight) term. Body weights were significantly greater in αERKO compared with WT mice beginning at 3 months of age and subsequently. Despite progressively greater body weight in αERKO mice compared with WT, average food consumption was similar and unchanged over time in the two groups (14.9 ± 0.8 and 14.8 ± 0.8 kcal/day in WT and αERKO, respectively), indicating greater feed efficiency in αERKO mice. SE refers to the overall standard deviation of the error term for each regression analysis.
Overall energy expenditure was significantly greater in WT vs. αERKO sibling male pairs (1.86 ± 0.08 vs. 1.65 ± 0.11 ml O2/min in WT and αERKO males, respectively; n = 7 and P < 0.05), corresponding to an 11% decrease in αERKO males. When expressed as a function of weight, energy expenditure in WT and αERKO sibling male pairs was 62.0 ± 2.6 vs. 50.0 ± 2.0 ml O2/kg per min, respectively. This 19% decrease reflects both the overall decrease in energy expenditure, as well as the much lower metabolic activity of fat, which is increased in αERKO males, relative to lean body mass.
Discussion
Based on both human and animal data, it is clear that E2 regulates WAT deposition in females (5, 6). The discovery of a second ER, ERβ, and expression of ERα and ERβ in WAT suggests both could potentially regulate WAT. Our results show that αERKO females have increased WAT, and that ERα plays a critical inhibitory role in the development and ultimate amount of female WAT. These findings suggest that increases in WAT after estrogen deficiency and decreases in WAT after estrogen replacement (3, 8) predominately reflect changes in signaling through ERα, although ERβ may play a role.
Estrogen treatment decreased adipocyte size in male rats (6), but it was unclear whether estrogen effects were direct or reflected changes in testosterone or other hormones. In addition, even if exogenous estrogen had direct effects on male WAT, this did not prove a role in normal WAT development. Our unexpected finding that αERKO males have increased WAT indicates that E2/ERα signaling is also important for normal development and function of WAT in males. The present results indicate that overall male WAT amounts, as well as adipocyte number and size, are altered in the absence of ERα. This appears to be a critical effect, as judged by the magnitude of WAT increases in αERKO mice. Furthermore, ERα appears to be equally important in both sexes, as shown by similar WAT increases in αERKO males and females.
Observed phenotypic effects on WAT in αERKO mice may not result solely from lack of ERα. Changes in ERα/ERβ ratios or the absence of heterodimer formation by ERα and ERβ (18) or other putative ERs (19) in αERKO mice could play a role in their obese phenotype. Similarly, increased circulating E2 levels in αERKO females (20) could produce increased signaling through ERβ or other ERs (19) and effects on WAT. However, this should not be a factor for increased WAT in αERKO males, because their E2 levels are similar to WT (20). Corticosterone levels are similar in juvenile and adult αERKO males (21), and testosterone, which is lipolytic like corticosterone, is increased in both αERKO males and females (20). Therefore, the obese phenotype in αERKO mice would not appear to result from changes in these hormones. However, changes in insulin levels, as discussed below, or other secondary hormonal effects could affect development of the obese phenotype in αERKO mice. The recent development of mice lacking either ERβ or both ERα and ERβ (22, 23) provides useful tools to establish the role of ERβ and ERα/ERβ interactions in regulating WAT.
Increased epididymal WAT is present by 30 days of age and becomes more pronounced with advancing age. Other fat pads in αERKO males showed increases equaling that in epididymal fat at later ages; weights of all three WAT depots in αERKO males were more than 100% greater than WT by 9–12 months of age. ER levels in young rats are higher in epididymal than perirenal or s.c. WAT, although in older animals ER levels in perirenal and s.c. WAT equaled or exceeded that of epididymal WAT (6). Thus, epididymal WAT may initially depend more on E2/ERα signaling, and ERα absence may cause increases in this WAT depot before perirenal and s.c. sites.
The increased WAT in αERKO mice involves both adipocyte hyperplasia and hypertrophy. In contrast, previously reported changes in WAT induced by ovariectomy and/or estrogen treatment predominately involved changes in adipocyte size (7). The increased adipocyte numbers in αERKO mice may result from changes in the differentiation and/or proliferation of the mesenchymal/preadipocyte/adipocyte lineage. Conversely, changes in WAT after ovariectomy and/or estrogen treatment (3, 6, 8) were observed in adults, when generation of new adipocytes is minimal, so these increases predominately reflect changes in size of existing adipocytes. Differences seen in αERKO compared with ovariectomized and/or estrogen-treated mice are in agreement with data that adult-onset obesity results from increased volume of existing adipocytes, whereas early-onset obesity reflects increased adipocyte number and size (24).
Weight of BAT is not altered in WT vs. αERKO males or females. However, estrogens affect BAT metabolism (3, 25), suggesting that there may be functional alteration in BAT of αERKO males or females even though no wet weight differences were apparent.
Both male and female αERKO mice have moderate insulin resistance and impaired glucose tolerance compared with WT, reflected in higher glucose and insulin after glucose challenge and their trend toward increased basal insulin levels. These findings are consistent with data that ovariectomy impairs glucose tolerance in mice, and that estrogen reverses this (9, 26). Similarly, estrogen replacement has beneficial effects on glucose tolerance in postmenopausal women (4, 11). Estrogens improve glucose tolerance and responsiveness to insulin in part through direct effects on insulin receptor levels in adipocytes (7). The altered glucose tolerance and insulin resistance in αERKO mice could be involved in development of obesity in both sexes.
The WAT changes in αERKO males and females represent a specific increase in this tissue. Although there are small increases in body and kidney weights in αERKO males and females, in agreement with a recent report indicating a 14% increase in adult body weight in αERKO females (27), these increases were far less than those in WAT and indicate that the increased WAT is not simply the result of increased body weight.
Our results indicate that the increased WAT deposition in αERKO males during adulthood is not accompanied by increased food consumption, despite reports that estrogen treatment in rodents depresses food consumption and rats show a transient hyperphagia after ovariectomy that contributes increased fat after ovariectomy (3). However, if hyperphagia is prevented by giving ovariectomized rats amounts of food equal to or even somewhat less than they consumed preovariectomy, they still develop increased fat. Likewise, hamsters show increased fat deposition after ovariectomy that is not accompanied by hyperphagia (3). Thus, hyperphagia is not essential for development of obesity in ovariectomized rats and hamsters, a finding consistent with our present results showing that obesity develops in αERKO males without a concomitant increase in food consumption.
The increased feed efficiency (increase in body weight/kcal eaten) and absence of increased energy intake in adult αERKO males suggested that decreased energy expenditure may be critical for the development of obesity in αERKO males, so this was assessed by indirect calorimetry. In contrast to the similar food consumption, energy expenditure in αERKO males was significantly reduced (11%) compared with WT males; reduced energy expenditure in the face of continued normal energy intake in the αERKO males may be a critical aspect of the mechanism by which obesity develops in these animals.
These results indicate that ERα may mediate the effects of E2 on energy expenditure and that E2/ERα signaling may be important for regulation of energy expenditure in males. Older human literature reported a decrease in basal metabolic rate in women after ovariectomy, and estrogens could reverse this (28). Similarly, an effect of E2 signaling on energy expenditure in female rodents was postulated for many years based on indirect evidence that rats and hamsters increased fat stores after ovariectomy without increased food consumption, suggesting postovariectomy energy expenditure was decreased (3). This hypothesis subsequently was supported by data derived from direct measurements of metabolism, which suggested a small increase in energy expenditure in ovariectomized rats in response to E2 (29, 30), and other findings that estrogen could increase rates of heat production and heat loss in ovariectomized rats (31). These results, in conjunction with the present data, suggest that E2 may act through ERα to increase energy expenditure in both males and females.
The energy expenditure data presented here represents the summation of both voluntary activity and basal metabolic activity. Stimulatory effects of estrogen on voluntary physical activity such as wheel running are known (32), and the magnitude of activities such as wheel running throughout the estrous cycle closely parallel E2 levels (3). Thus, our observed decrease in overall energy expenditure may reflect decreases in voluntary and/or basal metabolic activity in the αERKO males, and this is an area of active investigation.
The gamut of phenotypic and metabolic changes reported here for αERKO males and females parallel those in aromatase knockout (ArKO) mice (33), which lack aromatase cytochrome P450 and cannot produce endogenous estrogen (34). Insulin resistance and impaired glucose tolerance is seen in both male and female αERKO and ArKO mice. In addition, male and female ArKO mice become increasingly obese with age (33), and gonadal fat at 1 year of age in ArKO mice is doubled, similar to αERKO males. The lack of hyperphagia despite the observed obesity reported here for αERKO mice is also consistent with observations in ArKO mice. The similar effects in mice lacking ERα and those lacking the ligands for both ERα and ERβ suggest that ERα, rather than ERβ, is most critical for estrogen effects on WAT and metabolism.
The one known human male lacking ERα (12) had a body weight approximately 2 SD greater than normal. However, this individual also had increased height because of a lack of epiphysial plate fusion. Thus, continued growth may mitigate potential increases in WAT that might normally occur because of a lack of ERα. However, men and women lacking aromatase manifest truncal obsesity (13, 35). This and the insulin resistance and impaired glucose tolerance observed in both humans lacking ERα or aromatase and their murine counterparts emphasize that similar effects accompany loss of ERα in both species and strongly suggest ERα may regulate adipose tissue in men.
The role of estrogen and ERα in male reproductive and other organs has been unclear. Although ER are widely distributed in males, estrogen levels are much lower than in females. However, recent insights from both humans and mice lacking ERα or aromatase revealed that estrogen regulates efferent ductule function and germ cell development in male mice, protects against cardiovascular disease, has effects on lipid profiles and insulin resistance in men, and regulates male thymic maturation in male mice (reviewed in refs. 35 and 36). Our results suggesting a role for ERα in male metabolism and WAT development further illustrate estrogen's critical effects in the male.
In summary, our results show that lack of ERα produces large increases in WAT in both male and female mice and is accompanied by insulin resistance and glucose intolerance in both sexes. The increased WAT in αERKO males appears to result from decreased energy expenditure accompanied by continued normal energy intake, rather than hyperphagia. The phenotypic and metabolic changes in WAT and other tissue in αERKO males show that ERα plays a greater role in males than previously appreciated. The αERKO mouse may be a useful model to study factors regulating obesity and metabolism and the role of estrogen and ERα in this process.
Acknowledgments
We thank S. Yellayi, S. Smith, M. Borgstrom, and M. Hufford for technical assistance, D. Schaeffer and P. Constable for statistical analysis, and R. Gaskins and D. Buchanan for proofreading. This work was supported by National Institutes of Health Grants AG 15500 (P.S.C.) and ES 08272 (D.B.L.), and Animal Health and Disease Research Funds from the University of Illinois (P.S.C. and P.A.H.).
Abbreviations
- ER
estrogen receptor
- αERKO
ERα knockout
- WAT
white adipose tissue
- BAT
brown adipose tissue
- WT
wild type
- E2
estradiol 17-β
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Portions of this work were presented at the 32nd and 33rd Annual Meetings of the Society for the Study of Reproduction, July 31–Aug. 3, 1999, Pullman, WA, and July 15–18, 2000, Madison, WI, respectively.
References
- 1.Taubes G. Science. 1998;280:1367–1368. doi: 10.1126/science.280.5368.1367. [DOI] [PubMed] [Google Scholar]
- 2.Allison D B, Fontaine K R, Manson J E, Stevens J, VanItallie T B. J Am Med Assoc. 1999;282:1530–1538. doi: 10.1001/jama.282.16.1530. [DOI] [PubMed] [Google Scholar]
- 3.Wade G N, Gray J M. Physiol Behav. 1985;22:583–593. doi: 10.1016/0031-9384(79)90028-3. [DOI] [PubMed] [Google Scholar]
- 4.Tchernof A, Calles-Escandon J, Sites C K, Poehlman E T. Coron Artery Dis. 1998;9:503–511. doi: 10.1097/00019501-199809080-00006. [DOI] [PubMed] [Google Scholar]
- 5.Wade G N, Gray J M. Endocrinology. 1978;103:1695–1701. doi: 10.1210/endo-103-5-1695. [DOI] [PubMed] [Google Scholar]
- 6.Pederson S B, Borglum J D, Eriksen E F, Richelsen B. Biochim Biophys Acta. 1991;1093:80–86. doi: 10.1016/0167-4889(91)90141-j. [DOI] [PubMed] [Google Scholar]
- 7.Pederson S B, Borglum J D, Moller-Pederson T, Richelsen B. Mol Cell Endocrinol. 1992;85:13–19. doi: 10.1016/0303-7207(92)90120-u. [DOI] [PubMed] [Google Scholar]
- 8.Crandall D L, Busler D E, Novak T J, Weber R V, Kral J G. Biochem Biophys Res Commun. 1998;248:523–526. doi: 10.1006/bbrc.1998.8997. [DOI] [PubMed] [Google Scholar]
- 9.Bailey C J, Ahmed-Sorour H. Diabetologia. 1980;19:475–481. doi: 10.1007/BF00281829. [DOI] [PubMed] [Google Scholar]
- 10.Bailey C J, Matty A J. Horm Metab Res. 1972;4:266–270. doi: 10.1055/s-0028-1094063. [DOI] [PubMed] [Google Scholar]
- 11.Lindheim S R, Buchanan T A, Duffy D M, Vijod M A, Kojima T, Stanczyk F Z, Lobo R A. J Soc Gynecol Invest. 1994;1:150–154. doi: 10.1177/107155769400100210. [DOI] [PubMed] [Google Scholar]
- 12.Smith E P, Boyd J, Frank G R, Takahashi H, Cohen R M, Specker B, Williams T C, Lubahn D B, Korach K S. N Engl J Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 13.Morishima A, Grumbach M M, Simpson E R, Fisher C, Qin K. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 14.Lubahn D B, Moyer J S, Golding T S, Couse J F, Korach K S, Smithies O. Proc Natl Acad Sci USA. 1993;90:11162–11166. doi: 10.1073/pnas.90.23.11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodbell M. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- 16.Hadigan C, Miller K, Corcoran C, Anderson E, Basgoz N, Grinspoon S. J Clin Endocrinol Metab. 1999;84:1932–1937. doi: 10.1210/jcem.84.6.5738. [DOI] [PubMed] [Google Scholar]
- 17.Brooks G A, White T P. J Appl Physiol. 1978;45:1009–1015. doi: 10.1152/jappl.1978.45.6.1009. [DOI] [PubMed] [Google Scholar]
- 18.Cowley S M, Hoare S, Mosselman S, Parker M G. J Biol Chem. 1997;272:19858–19862. doi: 10.1074/jbc.272.32.19858. [DOI] [PubMed] [Google Scholar]
- 19.Das S K, Taylor J A, Korach K S, Paria B C, Dey S K, Lubahn D B. Proc Natl Acad Sci USA. 1997;94:12786–12791. doi: 10.1073/pnas.94.24.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 21.Yellayi S, Teuscher C, Woods J A, Welsh T H, Jr, Tung K S, Nakai M, Rosenfeld C S, Lubahn D B, Cooke P S. Endocrine. 2000;12:207–213. doi: 10.1385/endo:12:3:207. [DOI] [PubMed] [Google Scholar]
- 22.Krege J H, Hodgin J B, Couse J F, Enmark E, Warner M, Mahler J F, Sar M, Korach K S, Gustafsson J-A, Smithies O. Proc Natl Acad Sci USA. 1998;95:15677–15682. doi: 10.1073/pnas.95.26.15677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Couse J F, Hewitt S C, Bunch D O, Sar M, Walker V R, Davis B J, Korach K S. Science. 1999;286:2328–2331. doi: 10.1126/science.286.5448.2328. [DOI] [PubMed] [Google Scholar]
- 24.Hirsch J, Han P W. J Lipid Res. 1969;10:77–82. [PubMed] [Google Scholar]
- 25.Kemnitz J W, Glick Z, Bray G A. Pharmacol Biochem Behav. 1983;18:563–566. doi: 10.1016/0091-3057(83)90281-2. [DOI] [PubMed] [Google Scholar]
- 26.Ahmed-Sorour H, Bailey C J. Horm Res. 1980;13:396–403. doi: 10.1159/000179307. [DOI] [PubMed] [Google Scholar]
- 27.Vidal O, Lindberg M, Savendahl L, Lubahn D B, Ritzen E M, Gustafsson J A, Ohlsson C. Biochem Biophys Res Commun. 1999;265:569–571. doi: 10.1006/bbrc.1999.1711. [DOI] [PubMed] [Google Scholar]
- 28.Collett M E, Smith J T, Werternberger G E. Am J Obstet Gynecol. 1937;34:639–656. [Google Scholar]
- 29.Richard D. Am J Physiol. 1986;250:R245–R249. doi: 10.1152/ajpregu.1986.250.2.R245. [DOI] [PubMed] [Google Scholar]
- 30.Guyard B, Fricker J, Brigant L, Betoulle D, Apfelbaum M. Metabolism. 1991;40:529–533. doi: 10.1016/0026-0495(91)90236-p. [DOI] [PubMed] [Google Scholar]
- 31.Laudenslager M L, Wilkinson C W, Carlisle H J, Hammel H T. Am J Physiol. 1980;238:R400–R405. doi: 10.1152/ajpregu.1980.238.5.R400. [DOI] [PubMed] [Google Scholar]
- 32.Mook D G, Kenney N J, Roberts S, Nuissbaum A I, Rodier W I., III J Comp Physiol Psychol. 1972;81:198–211. doi: 10.1037/h0033526. [DOI] [PubMed] [Google Scholar]
- 33.Jones M E E, Thorburne A, Britt K L, Hewitt K N, Wreford N G, Proietto J, Oz O K, Leury B, Robertson K M, Yao S, Simpson E R. Proc Natl Acad Sci USA. 2000;97:12735–12740. doi: 10.1073/pnas.97.23.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher C R, Graves K H, Parlow A F, Simpson E R. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grumbach M M, Auchus R J. J Clin Endocrinol Metab. 1999;84:4677–4694. doi: 10.1210/jcem.84.12.6290. [DOI] [PubMed] [Google Scholar]
- 36.Cooke, P. S., Heine, P. A., Taylor, J. A. & Lubahn, D. B. (2000) Mol. Cell. Endocrinol., in press. [DOI] [PubMed]