Abstract
The aromatase-knockout (ArKO) mouse provides a useful model to examine the role that estrogens play in development and homeostasis in mammals. Lacking a functional Cyp19 gene, which encodes aromatase, the ArKO mouse cannot synthesize endogenous estrogens. We examined the adipose depots of male and female ArKO mice, observing that these animals progressively accumulate significantly more intraabdominal adipose tissue than their wild-type (WT) littermates, reflected in increased adipocyte volume at gonadal and infrarenal sites. This increased adiposity was not due to hyperphagia or reduced resting energy expenditure, but was associated with reduced spontaneous physical activity levels, reduced glucose oxidation, and a decrease in lean body mass. Elevated circulating levels of leptin and cholesterol were present in 1-year-old ArKO mice compared with WT controls, as were elevated insulin levels, although blood glucose levels were unchanged. Associated with these changes, a striking accumulation of lipid droplets was observed in the livers of ArKO animals. Our findings demonstrate an important role for estrogen in the maintenance of lipid homeostasis in both males and females.
Keywords: estrogen deficiency, obesity, insulin, cholesterol, leptin
Aromatase is encoded by the Cyp19 gene and catalyzes the final step in the biosynthesis of C18 estrogens from C19 steroids. The sexually dimorphic distribution of adipose tissue in humans has implicated sex steroids in the regulation of adiposity and distribution of fat depots. Thus, whereas premenopausal women tend to have a lower body or gynoid distribution of fat, men and postmenopausal women tend to have an upper body or android distribution of fat. This phenotype is associated with a greater risk of insulin-resistant diabetes, cardiovascular disease, and breast cancer (1). Estrogen insufficiency is thought to be largely responsible for the increase in adiposity during menopause because postmenopausal women who receive estrogen replacement therapy do not display the characteristic abdominal weight gain pattern usually associated with menopause (2). The role that estrogens play in lipid metabolism in the body is also highlighted by the fact that individuals of both sexes with natural mutations of the gene encoding aromatase, the enzyme responsible for estrogen biosynthesis, develop truncal obesity, insulin resistance, hypercholesterolemia, and hypertriglyceridemia (3–6).
We have recently developed a mouse model of estrogen insufficiency by targeted disruption of the aromatase gene: the aromatase-knockout (ArKO) mouse (7). In the course of these studies, we observed that the animals displayed a progressive increase in adiposity as compared with wild-type (WT) littermates. The aim of the present investigation was to characterize the obese phenotype of these animals in the expectation that this would throw light on the role of estrogens in lipid homeostasis.
Materials and Methods
Mice.
ArKO mice were generated by disrupting the Cyp19 gene as described (7). Heterozygous males and females were bred to produce WT and homozygous-null offspring. Mice were genotyped by PCR as described (8). Animals were maintained under specific pathogen-free conditions and had unlimited access to drinking water and a mouse diet containing 15% of calories as fat, 20% of calories as protein, and 65% of calories as carbohydrate (manufactured by Glen Forrest Stockfeeders, Glenn Forrest, Western Australia).
Tissue Collection and Histology.
Mice were anesthetized with pentobarbitone sodium (Nembutal, Rhone Merieux Australia, Pinkenba, Australia) administered by i.p. injection (60 mg per kg of body weight). Thirty minutes later, trunk blood was collected after decapitation. Blood was allowed to clot. The serum was separated and stored at −20°C. Gonadal fat pads and infrarenal fat pads were removed and the wet mass was measured. Infrarenal white adipose tissue lay behind the kidneys next to the abdominal wall in a well-defined depot and did not include the fat surrounding the kidneys. Liver samples were collected at the same time. Samples of gonadal fat and liver were immersion-fixed in Bouins fluid, then stored in 70% alcohol at 4°C. Samples were embedded with a random orientation in paraffin and sliced into 10 μm sections. Sections were stained with hematoxylin, counterstained with eosin, then coverslipped with DPX (BDH).
Estrogen Replacement.
ArKO and WT female mice were implanted with 21-day release 17β-estradiol or placebo pellets (0.05 mg of 17β-estradiol per pellet; Innovative Research of America) at 7 weeks of age. After 21 days, tissues were collected as described above.
Adipocyte Volume Measurement.
Adipocyte volume was determined by measuring point sampled intercepts on the paraffin sections (9). Intercepts were measured in two directions by using castgrid Version 1.10 (Olympus, New Hyde Park, NY) on an Olympus BX50 microscope. Sections were exhaustively sampled by using a systematic uniform random scheme with the x and y shifts set at 50 μm. Approximately 100 measurements of volume were determined across the tissue sections derived from each sample.
Body Fat Composition by Proton Magnetic Resonance.
Body fat composition was determined at the Southwestern Medical Center and the Howard Florey Institute. Whole-body proton MRI was used to determine percent body fat as described (10). Briefly, animals were placed in a coil which covered the entire body, then inserted into a 4.7-T Oxford magnet. Proton spectra were obtained and resolved into the water and lipid resonances. The areas of each were quantified by using the NMR-1 software program (Tripos Associates, St. Louis). The percent of fat is calculated as a ratio of the fat peak area to that of water.
Body Composition Analysis.
Methods for the chemical analysis of mouse body composition were adapted from those described (11).
Measures of Energy Balance.
Energy intake, spontaneous physical activity, and resting energy expenditure were measured in female ArKO and WT mice. Daily caloric consumption was measured over a 4-day period. During this time, mice were individually housed in Perspex boxes so that daily spontaneous physical activity levels could be simultaneously measured by using an infrared light beam monitor (Columbus Instruments, Columbus, OH). After these measurements were completed, and after a 2-h fast, rates of resting energy expenditure, glucose oxidation, and fat oxidation were measured by using indirect calorimetry (Columbus Instruments) as described (12).
Measurement of Serum Glucose, Insulin, Leptin, Cholesterol, Triglycerides, and High Density Lipoprotein (HDL).
Blood serum was analyzed for glucose, insulin, leptin, cholesterol, triglycerides, and HDL. The glucose oxidase method was used to determine serum glucose by using a Yellow Springs Instruments glucose analyzer. Serum leptin and insulin were determined by radioimmunoassays (Linco Research Immunoassay, St. Charles, MO, and Amersham Pharmacia, respectively). Levels of cholesterol, triglycerides, and HDL were quantified by using the appropriate Dimension clinical chemistry system for each—Cholesterol Flex, Triglyceride Flex, and Automated HDL Cholesterol kits, respectively (Dade Behring, Newark, DE).
Statistical Analysis.
Data are expressed as mean ± SEM. Comparisons between ArKO and WT mice were made by using the Student's two-tailed t test (Microsoft EXCEL 97). All experiments conformed to the National Health and Medical Research Council (Australia) ethics code of practice.
Results
Body and Fat Pad Masses.
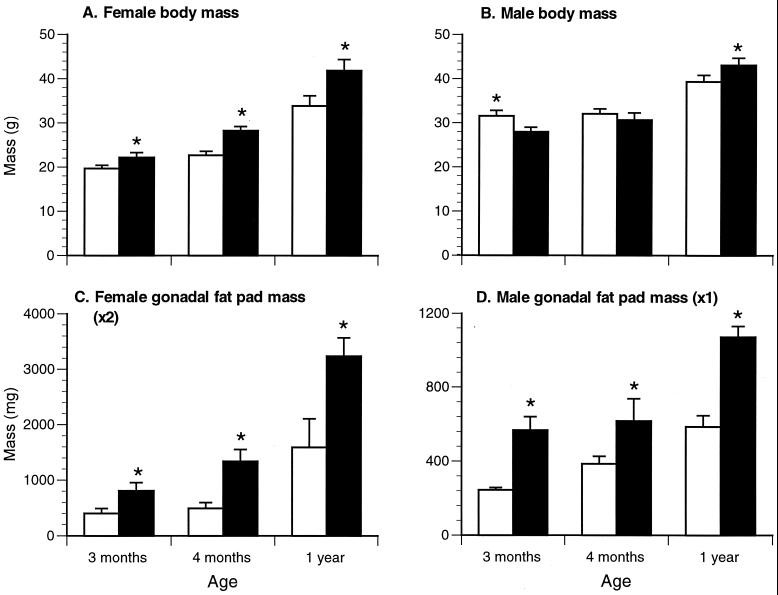
Adult female ArKO mice were significantly heavier and had significantly larger gonadal and infra-renal fat pads than WT littermates from 3 months of age onward (Fig. 1 A and C and Table 1). Male ArKO mice also had significantly heavier gonadal fat pads than WT males from 3 months of age and heavier infrarenal fat pads from 4 months of age (Fig. 1 B and D and Table 1). However, their body weight failed to show a significant increase until 1 year of age.
Figure 1.
Body mass and gonadal fat pad mass for ArKO (filled bars) and WT (empty bars) mice. (A) Female body mass. (B) Male body mass. (C) Female gonadal fat pad mass (two pads). (D) Male gonadal fat pad (pad) mass. Minimum six animals per group. *, P ≤ 0.05.
Table 1.
Infrarenal fat pad masses
| Mice | Wet weight, mg
|
||
|---|---|---|---|
| 3 months | 4 months | 1 year | |
| Females | |||
| ArKO | 75.4 ± 10.3 (7)* | 96.3 ± 26.3 (3)* | 190.2 ± 30.5 (23)* |
| WT | 44.1 ± 8.0 (22) | 42.0 ± 9.0 (17) | 96.1 ± 27.8 (8) |
| Males | |||
| ArKO | 93.1 ± 20.0 (13) | 206.9 ± 17.0 (8)* | 213.2 ± 17.6 (11)* |
| WT | 115.6 ± 16.4 (13) | 132.4 ± 41.5 (3) | 105.8 ± 8.3 (11) |
Results are presented as mean ± SEM (n). *, At least P < 0.05 compared to WT.
Estrogen Replacement.
Administration of exogenous 17β-estradiol for 21 days to 7-week-old female ArKO mice restored their fat depots to masses comparable to, or less than, those of WT littermates (Table 2). A comparison between placebo- and estradiol-treated ArKO animals, for both gonadal and infra-renal fat pad masses, showed that the reduction in mass was statistically significant.
Table 2.
Effect of estrogen replacement on fat depot masses
| Mice and treatment | Wet weight, mg
|
|
|---|---|---|
| Gonadal fat pad | Infrarenal fat pad | |
| ArKO + placebo | 1033.2 ± 53.4 | 95.1 ± 7.6 |
| WT + placebo | 612.3 ± 373.6* | 49.0 ± 19.7* |
| ArKO + E2 replacement | 228.1 ± 13.8* | 19.3 ± 1.3* |
Results are presented as mean ± SEM (n = 3 for all groups). *, At least P < 0.05 compared to ArKO placebo treatment.
MRI.
In agreement with the fat pad data, MRI showed that ArKO males and females have a significantly greater percentage of adipose tissue than their WT littermates from as early as 10 weeks, through 1 year of age (Table 3). By 1 year of age, both knockout and WT females had more body fat than males. The considerable increase in body fat measured in 1-year-old animals reflects the significant increase in fat pad mass observed between 4 months and 1 year of age.
Table 3.
Percent adipose tissue as determined by MRI
| Mice | %
adipose tissue
|
|
|---|---|---|
| 10 weeks | 1 year | |
| Females | ||
| ArKO | 17.6 ± 4.4 (5)* | 64.3 ± 11.0 (19)* |
| WT | 4.9 ± 1.0 (5) | 42.1 ± 6.7 (9) |
| Males | ||
| ArKO | 15.2 ± 2.3 (5)* | 40.3 ± 3.8 (13)* |
| WT | 7.3 ± 1.7 (5) | 29.5 ± 3.7 (16) |
Results are presented as mean ± SEM (n). *, At least P < 0.05 compared to WT.
Body Composition Analysis.
Despite the marked increase in adiposity at 3 months of age, the male ArKO animals failed to show a corresponding increase in body weight and the female ArKO mice showed only a mild increase in body weight, suggesting there was a concomitant decrease in lean mass. To assess this, body composition was analyzed (results presented in Table 4). It can be seen that the increase in fat mass was accompanied by a significant decrease in lean mass in the ArKO mice.
Table 4.
Body composition analysis on 15-week-old female mice
| Mice | Composition, %
|
||
|---|---|---|---|
| Lean | Fat | Ash | |
| ArKO | 75.7 ± 2.7* | 20.0 ± 2.8* | 4.3 ± 0.5 |
| WT | 83.4 ± 1.2 | 12.2 ± 1.4 | 4.5 ± 0.3 |
Results are presented as mean ± SEM (n = 8 per group). *, P < 0.03 compared to WT.
Adipocyte Volume.
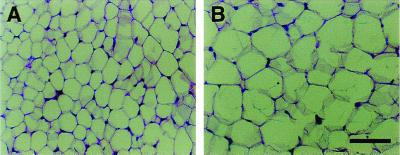
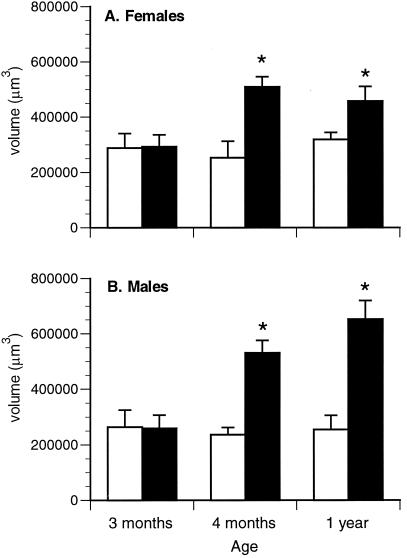
Adipose tissue accretion may be due to an increase in the number of adipocytes or an increase in the volume of individual adipocytes, or both. Visual assessment of gonadal fat tissue from 1-year-old male animals indicated that adipocyte size was larger in ArKO animals than in WT animals (Fig. 2). To evaluate adipocyte volume, stereological analyses were conducted. As shown in Fig. 3, adipocyte volume is unchanged over time in WT control animals, and cell volume appears to be very similar between WT males and females. At 3 months of age, adipocyte volume was the same between knockout and control animals for both genders. By 4 months of age, however, there was a significant increase in the volume of individual adipocytes in the gonadal fat pads of male and female ArKO mice when compared to WT mice. This increase in cell volume was maintained through 1 year of age.
Figure 2.
Gonadal fat pads from 1-year-old male mice were fixed, sectioned, and stained with hematoxylin and eosin. (A) WT. (B) ArKO. (Bar represents 50 μm.)
Figure 3.
Adipocyte volume data for female (A) and male (B) mice. Empty bars, WT; filled bars, ArKO. Minimum five animals per group. *, P ≤ 0.05.
Energy Balance.
The female mice used for the energy balance studies were the same as those used for the body composition analysis described above. Body weights of the 3.5-month-old female ArKO mice (n = 10) and WT mice (n = 10) were similar (25.6 ± 1.3 g in ArKO and 24.7 ± 0.7 g in WT), whereas lean body mass was lower and fat mass was higher in ArKO mice (Table 4). Increased adiposity in these ArKO mice was not associated with hyperphagia. In fact, there was a strong trend for food intake to be lower in ArKO mice as compared to WT mice (15.7 ± 0.8 vs. 19.1 ± 1.1 kcal/d, P = 0.06). In younger female mice, studied at 5 weeks of age, this phenomenon was also observed (14.5 ± 0.3 vs. 19.9 ± 2.1 kcal/d, P = 0.03, n = 6). Adiposity in the older female ArKO mice was, however, related to reduced spontaneous physical activity, ArKO mice being half as active as WT mice (43,285 ± 4,205 vs. 93,839 ± 15,925 ambulatory movements per day, P < 0.01). A similar trend was observed in the younger mice, although this was not significant (45,577 ± 7,516 vs. 63,058 ± 11,149 ambulatory movements per day). Resting energy expenditure was not significantly lower in ArKO mice than in WT mice (5.99 ± 0.33 vs. 6.68 ± 0.56 kcal/d), and fat oxidation rates were not significantly lower in the ArKO mice than in WT mice (0.34 ± 0.02 vs. 0.32 ± 0.03 mg/min). Resting energy expenditure and fat oxidation remained similar in ArKO and WT mice when normalized for fat-free mass or fat mass, respectively. Glucose oxidation rates, however, were 59% lower in the ArKO mice (0.11 ± 0.03 vs. 0.26 ± 0.05 mg/min, P < 0.02). This difference in glucose oxidation was still present when normalized for lean body mass.
Serum Cholesterol, Triglycerides, and HDL.
At 3–4 months of age, there was no difference in serum cholesterol, triglycerides, and HDL between knockout and WT females or between knockout and WT males (Table 5).
Table 5.
Serum lipid profile of ArKO and WT mice
| Mice | Lipid, mmol/liter
|
||
|---|---|---|---|
| Cholesterol | Triglycerides | HDL | |
| Females | |||
| 3–4 months | |||
| ArKO | 3.63 ± 0.41 (6) | 1.40 ± 0.22 (6) | 2.28 ± 0.26 (6) |
| WT | 3.25 ± 0.15 (12) | 1.27 ± 0.11 (12) | 2.20 ± 0.12 (12) |
| 1 year | |||
| ArKO | 4.34 ± 0.63 (5)* | 2.28 ± 0.57 (5) | 2.92 ± 0.33 (5)* |
| WT | 2.74 ± 0.28 (5) | 2.23 ± 0.49 (5) | 1.77 ± 0.18 (5) |
| Males | |||
| 3–4 months | |||
| ArKO | 4.29 ± 0.22 (16) | 2.85 ± 0.35 (16) | 1.57 ± 0.10 (16) |
| WT | 4.08 ± 0.25 (23) | 2.38 ± 0.22 (23) | 1.75 ± 0.19 (23) |
| 1 year | |||
| ArKO | 4.79 ± 0.73 (7)* | 3.27 ± 0.39 (7)* | 2.84 ± 0.33 (7)* |
| WT | 3.39 ± 0.29 (8) | 2.08 ± 0.43 (8) | 2.10 ± 0.26 (8) |
Results are presented as mean ± SEM (n). *, At least P < 0.05 compared to WT.
However, 1-year-old female and male ArKO mice had significantly elevated cholesterol levels compared with gender-matched WT control littermates (P = 0.02 and P = 0.04, respectively). Interestingly, a between-gender comparison at 3–4 months of age revealed that cholesterol levels were significantly lower in female WT mice compared with male WT mice (P = 0.004), and although a similar trend was observed between ArKO female and male mice, the difference was not significant.
At 1 year of age there was no difference in circulating triglyceride levels between ArKO and WT females. However, triglyceride levels were significantly elevated in 1-year-old ArKO vs. WT males (P = 0.03). A between-gender comparison at 3–4 months of age showed that ArKO and WT female mice had lower circulating levels of triglycerides (ArKO, P = 0.001; WT, P < 0.001), compared with age-matched male mice of the same genotype.
By 1 year of age, ArKO females and males had significantly elevated levels (P = 0.008 and P = 0.05, respectively) of circulating HDL compared with gender-matched WT mice. A between-gender comparison at 3–4 months of age showed that ArKO and WT female mice had elevated circulating levels of HDL (ArKO, P = 0.002; WT, P = 0.03), compared with male mice of the same genotype.
Serum Insulin and Glucose.
Insulin levels were the same for ArKO and WT males at 4 months of age [ArKO, 5.98 ± 1.00 milliunits (mU)/liter (n = 3); WT, 5.26 ± 0.75 mU/liter (n = 3); mean ± SEM]. By 1 year of age, however, ArKO males had significantly elevated levels of insulin compared with WT mice [47.14 ± 12.46 mU/L (n = 6) vs. 12.42 ± 3.27 mU/L (n = 5), mean ± SEM, P = 0.02]. Glucose levels, on the other hand, when measured in 1-year-old males, were unchanged [ArKO, 8.52 ± 1.56 mmol/ liter (n = 3); WT, 8.61 ± 2.02 mmol/liter (n = 3); mean ± SEM]. Glucose levels were not measured in 4-month-old animals.
Serum Leptin.
Circulating leptin levels were significantly elevated in 4-month-old and 1-year-old male and female ArKO mice compared with their WT littermates (Table 6). An age-related increase in leptin concentration is evident in both ArKO and WT female mice.
Table 6.
Serum leptin levels in ArKO and WT mice
| Mice | Leptin,
ng/ml
|
|
|---|---|---|
| Females | Males | |
| 4 months | ||
| ArKO | 8.18 ± 0.78 (5)* | 8.79 ± 1.83 (6)* |
| WT | 2.92 ± 0.68 (5) | 3.81 ± 1.00 (7) |
| 1 year | ||
| ArKO | 19.86 ± 4.90 (6)*† | 8.47 ± 1.85 (7)* |
| WT | 6.19 ± 2.33 (4)* | 4.89 ± 0.72 (8) |
Results are presented as mean ± SEM (n). *, at least P < 0.05 compared to age-matched WT mice;
, at least P < 0.05 compared to 4-month-old, genotype-, and sex-matched mice.
Fatty Liver.
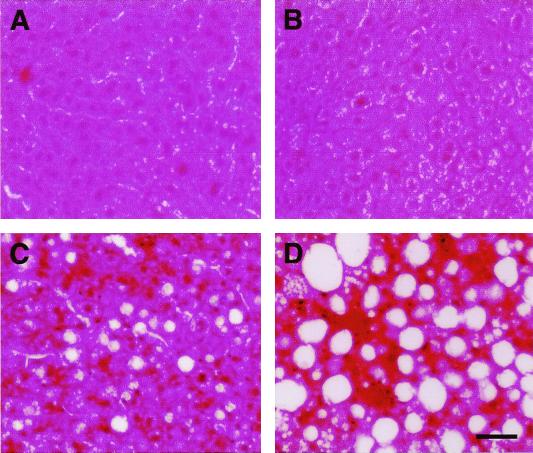
General inspection of internal organs at necropsy revealed that in 1-year-old ArKO animals the liver was often larger and paler in color than that of WT littermates. After fixation, sectioning, and staining, the livers of 3-month-old and 1-year-old ArKO animals were observed to have a greater accumulation of lipid droplets than WT control animals (Fig. 4).
Figure 4.
(A and B) Sectioned livers of 3-month-old (A) and 1-year-old (B) WT males. (C and D) Sectioned livers of 3-month-old (C) and 1-year-old (D) ArKO males. (Bar represents 50 μm.)
Discussion
The present studies reinforce and extend the concept that estrogens play an important role in the regulation of adiposity as a function of age in both males and females. Estrogens have important roles in adipose tissue, within the central nervous system, in bone, and in other extragonadal sites (13). The present study has shown that ArKO mice of both sexes with estrogen insufficiency develop a progressive increase in adiposity due to accumulation of intraabdominal fat. This was associated with an increase in adipocyte volume, hyperleptinemia, hyperinsulinemia, and hypercholesterolemia. However, unlike many other rodent models of obesity, excess body fat in the estrogen-insufficient ArKO mice was not due to hyperphagia or reduced resting energy expenditure, but was associated with decreased lean mass and reduced spontaneous physical activity. The obese phenotype is likely to be due to estrogen insufficiency rather than testosterone excess because 17β-estradiol treatment normalized body fat content in the ArKO mice, and the obese phenotype is similar in male and female ArKO mice despite their greatly different testosterone levels. Furthermore, a similar phenotype of fat accumulation is observed in estrogen-deficient ovariectomized rats, which cannot synthesize androgens (14).
An increase in adiposity and a decrease in lean mass in the ArKO mice suggests that estrogen affects nutrient partitioning. There are several possible biochemical mechanisms by which estrogen may have this effect. For example, oophorectomy is known to impair muscle glucose uptake and storage (15) in rats, which could decrease lean body mass. This decrease in glucose utilization by muscle would make more glucose carbon available for lipogenesis and could promote fat deposition. Muscle is responsible for most postprandial glucose uptake, so this decrease in muscle glucose uptake would also be expected to reduce whole body glucose oxidation and induce insulin resistance, as we have observed in the older ArKO mice. Alternatively, altered nutrient partitioning in the estrogen-insufficient ArKO mice may be due to impaired uncoupling protein function. Hope et al. (16) have shown decreased UCP2 mRNA expression in skeletal muscle but not adipose tissue of ovariectomized dunnarts (Sminthopsis crassicaudata) compared with sham-operated animals. This may reduce the utilization of nutrients specifically in muscle, reducing lean body mass and increasing adipose tissue mass.
The reduced spontaneous physical activity we found in the ArKO mice may also be partly responsible for the increase in fat pad mass. It is of interest that reduced physical activity is also reported to be a feature of estrogen deficiency in both ovariectomized animals (14) and postmenopausal women (17). Reduced physical activity in the ArKO mice may be secondary to the increase in fat mass and decrease in lean body mass seen in these mice. However, it is also possible that this inactivity is caused by an effect of estrogen insufficiency on the function of the central nervous system in these mice.
The ArKO mice had circulating leptin levels which were 2- to 3-fold higher than those of the WT mice from 4 months of age. This observation is not surprising, given the established association between obesity and increased production of leptin in most human subjects (18) and most animal models of obesity (19–22). Leptin regulates body fat predominantly by decreasing food intake (23). The fact that food intake is reduced in our ArKO mice suggests that these mice are responding normally to leptin. This fits with data showing that sensitivity to an intracerebroventricular dose of leptin is normal in obese ovariectomized mice, which have 59% lower estrogen levels than intact mice (24).
In older ArKO animals, the adiposity was associated with hypercholesterolemia and, at least in males, hypertriglyceridemia. The hypercholesterolemia was reflected in elevated HDL. Two aromatase-deficient men with mutations in the human CYP19 gene have also been reported to have altered circulating lipid profiles (4, 5). Blood serum levels of cholesterol, low density lipoprotein (LDL), and triglycerides were elevated in both men, but the concentration of HDL was decreased. Treatment with estradiol restored these parameters closer to normal limits. The different HDL response in aromatase-deficient mice and humans is likely due to the fact that the dominant lipoprotein involved in delivery of cholesterol to extrahepatic tissues and the liver (25, 26) is HDL in rodents, whereas LDL serves this role in humans. Hence it is not surprising that elevated serum cholesterol in mice is reflective, at least in part, of elevated HDL. The action of estrogens to lower circulating cholesterol in the form of LDL levels in humans has been implicated in their cardioprotective action (27). Nevertheless, oral estrogen increases serum triglyceride levels (27). This increase has been attributed to the “first pass” effect of oral administration exposing the liver to high concentrations of estrogen, because it is not seen with parenteral administration (28). Thus, the aromatase-deficiency phenotype and the results of parenteral administration would suggest that estrogens at circulating physiological concentrations do not increase serum triglycerides, and may in fact decrease them.
The observation that ArKO mice develop a fatty liver phenotype may be related to the observed hypercholesterolemia. One of the ways in which estrogen is believed to lower circulating cholesterol is by increasing the level of LDL receptor expression in the liver, thus enhancing LDL uptake (29). Moreover, mice deficient in the oxysterol receptor LXR (LXR−/−) fail to induce transcription of cholesterol 7α-hydroxylase (Cyp7a), the rate-limiting enzyme in bile acid synthesis, and accumulate cholesterol in the liver and have marked hypercholesterolemia (30). It has been reported that estrogen and progesterone increase the activity of cholesterol 7α-hydroxylase (31), so it is conceivable that both the fatty liver and hypercholestrolemia in the ArKO mice are due to a reduction in bile acid synthesis secondary to estrogen deficiency. Concomitant with a possible perturbation in this pathway, Nemoto et al. (32) report impairment in hepatocellular fatty acid β-oxidation in an aromatase-deficient mouse model they have independently generated. Analyses revealed a decrease in both mRNA expression and activities of specific enzymes required in fatty acid β-oxidation. Evidently, estrogens play multiple roles in lipid homeostasis, and are crucially involved not only in adipose depot accretion, but also in lipid turnover in the liver.
Associated with both increasing age and fat accumulation was a 4-fold increase in circulating levels of insulin, glucose levels remaining unchanged. Normoglycemia at the expense of hyperinsulinemia is suggestive of insulin resistance in 1-year-old ArKO mice. Elevated insulin levels are also found in humans with aromatase deficiency (3–5). Insulin resistance is a characteristic of obesity. The mechanism underlying this is unclear, but it appears to involve elevated free fatty acids and tumor necrosis factor α (33, 34).
Evidence that estrogen action plays an important role in body fat deposition is provided not only by these studies on the ArKO mouse, but also by two other rodent models of estrogen deprivation. Ovariectomized rats show an increase in body mass and fat deposition (14), as do α-estrogen receptor knockout (αERKO) mice (35). β-Estrogen receptor knockout (βERKO) mice, on the contrary, do not accumulate excess adipose tissue (36). This observation suggests that estrogen action, in terms of accumulation of fat, is mediated via ERα rather than ERβ, which is consistent with reports by ourselves and others that ERα, but not ERβ, is present in adipose tissue (ref. 36, ‡‡).
At present it is not known whether the increase in abdominal adiposity resulting from estrogen deficiency reflects direct actions of estrogens on the adipose depots or central actions or both. Estrogen is known to have direct effects on adipose tissue, e.g., oophorectomy decreases lipolysis in adipose tissue (37) of rats and estradiol treatment lowers fatty acid synthesis and increases lipolysis in fat cells (38). However, estrogen may also be involved in the central regulation of body weight, e.g., estrogen deficiency is known to increase hypothalamic neuropeptide Y expression (39) and decrease hypothalamic corticotropin-releasing factor immunoreactivity (40). Alternatively, estrogen may centrally regulate body weight by enhancing glucose uptake into the brain (41), a process that may facilitate recognition of the fed state. The fact that estrogen receptors are expressed within the central nervous system makes it possible that obesity in the ArKO and αERKO mice (35) is due, at least in part, to impaired signaling of estrogen through the α-estrogen receptor found in the hypothalamus of the brain. Further investigations are needed to unravel the cellular and biochemical mechanisms underlying the physiological phenomena reported here.
Acknowledgments
We thank Ms. Sue Panckridge, Ms. Fahi Nowniaz, and Mr. Hong Wang for assistance with figure preparation, chemical body composition analysis, and MRI scanning, respectively. This work was supported by U.S. Public Health Service Grant R37-AG 08174, National Health and Medical Research Council (Australia) Grant 981126, and the Victorian Breast Cancer Research Consortium.
Abbreviations
- ArKO
aromatase-knockout
- WT
wild-type
- HDL
high density lipoprotein
- LDL
low density lipoprotein
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Murata, Y., Boon, W. C., Cox, V., Oz, O. K., Thorburn, A., Proietto, J., Simpson, E. R. & Jones, M. E. E., U.S. Endocrine Society 81st Annual Meeting, June 12–15, 1999, San Diego, CA, abstr. P2–198.
References
- 1.Bouchard C, Depres J-P, Mauriege P. Endocr Rev. 1992;14:72–93. doi: 10.1210/edrv-14-1-72. [DOI] [PubMed] [Google Scholar]
- 2.Gambacciani M, Ciaponi M, Cappagli B, Piaggesi L, De Simone L, Orlandi R, Genazzani A R. J Clin Endocrinol Metab. 1997;82:414–417. doi: 10.1210/jcem.82.2.3735. [DOI] [PubMed] [Google Scholar]
- 3.Morishima A, Grumbach M M, Simpson E R, Fisher C, Qin K. J Clin Endocrinol Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- 4.Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach K, Simpson E R. New Engl J Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- 5.Bilezikian J P, Morishima A, Bell J, Grumbach M M. N Engl J Med. 1998;339:599–603. doi: 10.1056/NEJM199808273390905. [DOI] [PubMed] [Google Scholar]
- 6.Conte F A, Grumbach M M, Ito Y, Fisher C R, Simpson E R. J Clin Endocrinol Metab. 1994;78:1287–1292. doi: 10.1210/jcem.78.6.8200927. [DOI] [PubMed] [Google Scholar]
- 7.Fisher C R, Graves K H, Parlow A F, Simpson E R. Proc Natl Acad Sci USA. 1998;95:6965–6970. doi: 10.1073/pnas.95.12.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson K, O'Donnell L, Jones M E E, Meachem S J, Boon W C, Fisher C R, Graves K H, McLachlan R I, Simpson E R. Proc Natl Acad Sci USA. 1999;96:7986–7991. doi: 10.1073/pnas.96.14.7986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gundersen H J G, Bagger P, Bendtsen T F, Evans S M, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard J R, Pakkenberg B, et al. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 10.Stein D T, Babcock E E, Malloy C R, McGarry J D. Int J Obes. 1995;19:804–810. [PubMed] [Google Scholar]
- 11.Cunniff P, editor. Association of Analytical Chemists: Official Methods of Analysis of AOAC International. 16th Ed. Arlington, VA: AOAC International; 1995. [Google Scholar]
- 12.Hefferman M A, Jiang W J, Thorburn A W, Ng F M. Am J Physiol Endocrinol Metab. 2000;279:E501–E507. doi: 10.1152/ajpendo.2000.279.3.E501. [DOI] [PubMed] [Google Scholar]
- 13.Simpson E R, Rubin G, Clyne C, Robertson K, O'Donnell L, Jones M, Davis S. Trends Endocrinol Metab. 2000;11:184–188. doi: 10.1016/s1043-2760(00)00254-x. [DOI] [PubMed] [Google Scholar]
- 14.Wade G N. Physiol Behav. 1972;8:523–534. doi: 10.1016/0031-9384(72)90340-x. [DOI] [PubMed] [Google Scholar]
- 15.Rincon J, Holmang A, Wahlstrom E O, Lonnroth P, Bjorntorp P, Zierath J R, Wallberg-Henriksson H. Diabetes. 1996;45:615–621. doi: 10.2337/diab.45.5.615. [DOI] [PubMed] [Google Scholar]
- 16.Hope P J, Turnbull H, Breed W, Morley J E, Horowitz M, Wittert G A. Physiol Behav. 2000;69:463–470. doi: 10.1016/s0031-9384(99)00264-4. [DOI] [PubMed] [Google Scholar]
- 17.Poehlman E T, Tchernof A. Coronary Artery Dis. 1998;9:799–803. [PubMed] [Google Scholar]
- 18.Hamilton B S, Paglia D, Kwan A Y, Deitel M. Nat Med. 1995;1:953–956. doi: 10.1038/nm0995-953. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman J M. Nature (London) 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa Y, Masuzaki H, Isse N, Okazaki T, Mori K, Shigemoto M, Satoh N, Tamura N, Hosoda K, Yoshimasa Y, et al. J Clin Invest. 1995;96:1647–1652. doi: 10.1172/JCI118204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins S, Surwit R S. J Biol Chem. 1996;271:9437–9440. doi: 10.1074/jbc.271.16.9437. [DOI] [PubMed] [Google Scholar]
- 22.Funahashi T, Shimomura I, Hiraoka H, Arai T, Takahashi M, Nakamura T, Nozaki S, Yamashita S, Takemura K, Tokunaga K, et al. Biochem Biophys Res Commun. 1995;211:469–475. doi: 10.1006/bbrc.1995.1837. [DOI] [PubMed] [Google Scholar]
- 23.Farooqi I S, Jebb S A, Langmack G, Lawrence E, Cheetham C H, Prentice A M, Hughes I A, McCarnish M A, O'Rahilly S. N Engl J Med. 1999;341:879–915. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- 24.Pelleymounter M A, Baker M B, McCaleb M. Am J Physiol. 1999;276:E955–E963. doi: 10.1152/ajpendo.1999.276.5.E955. [DOI] [PubMed] [Google Scholar]
- 25.Ueda Y, Royer L, Gong E, Zhang J, Cooper P N, Francome O, Rubin E M. J Biol Chem. 1999;274:7165–7171. doi: 10.1074/jbc.274.11.7165. [DOI] [PubMed] [Google Scholar]
- 26.Trigatti B, Rayburn H, Vinalo M, Braun A, Miettinen H, Penman M, Hertz M, Schrenzel M, Amigo L, Rigotti A, et al. Proc Natl Acad Sci USA. 1999;96:9322–9327. doi: 10.1073/pnas.96.16.9322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darling G M, Johns J A, McCloud P I, Davis S R. N Engl J Med. 1997;337:595–601. doi: 10.1056/NEJM199708283370903. [DOI] [PubMed] [Google Scholar]
- 28.Davis S R, McCloud P I, Strauss B J, Burger H G. Maturitas. 1995;21:227–236. doi: 10.1016/0378-5122(94)00898-h. [DOI] [PubMed] [Google Scholar]
- 29.Kovanen P T. Am Heart J. 1987;113:464–469. doi: 10.1016/0002-8703(87)90615-6. [DOI] [PubMed] [Google Scholar]
- 30.Peet D J, Turley S D, Ma W, Janowski B A, Lobaccaro J A, Hammer R E, Mangelsdorf D J. Cell. 1998;93:693–704. doi: 10.1016/s0092-8674(00)81432-4. [DOI] [PubMed] [Google Scholar]
- 31.Chico Y, Fresnedo O, Botham K, Lacort M, Ochoa B. Exp Clin Endocrinol. 1996;104:137–144. doi: 10.1055/s-0029-1211435. [DOI] [PubMed] [Google Scholar]
- 32.Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. J Clin Invest. 2000;105:1819–1825. doi: 10.1172/JCI9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger R H. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 34.Hotamisligil B S, Spiegelman B M. Diabetes. 1994;43:1271–1278. doi: 10.2337/diab.43.11.1271. [DOI] [PubMed] [Google Scholar]
- 35.Heine P A, Taylor J A, Lubahn D B, Cooke P S. Proc Natl Acad Sci USA. 2000;97:12729–12734. doi: 10.1073/pnas.97.23.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Couse J F, Korach K S. Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 37.Darimont C, Delansorne R, Paris J, Ailhaud G, Negrel R. Endocrinology. 1997;138:1092–1096. doi: 10.1210/endo.138.3.4984. [DOI] [PubMed] [Google Scholar]
- 38.Hansen F M, Fahmy N, Nielsen J H. Acta Endocrinol. 1980;95:566–570. doi: 10.1530/acta.0.0950566. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu H, Ohtani K, Kato Y, Tanaka Y, Mori M. Neurosci Lett. 1996;204:81–84. doi: 10.1016/0304-3940(96)12322-3. [DOI] [PubMed] [Google Scholar]
- 40.Haas D A, George S R. Brain Res Bull. 1988;20:361–367. doi: 10.1016/0361-9230(88)90065-2. [DOI] [PubMed] [Google Scholar]
- 41.Bishop J, Simpkins J W. Brain Res Bull. 1995;36:315–320. doi: 10.1016/0361-9230(94)00208-i. [DOI] [PubMed] [Google Scholar]