Abstract
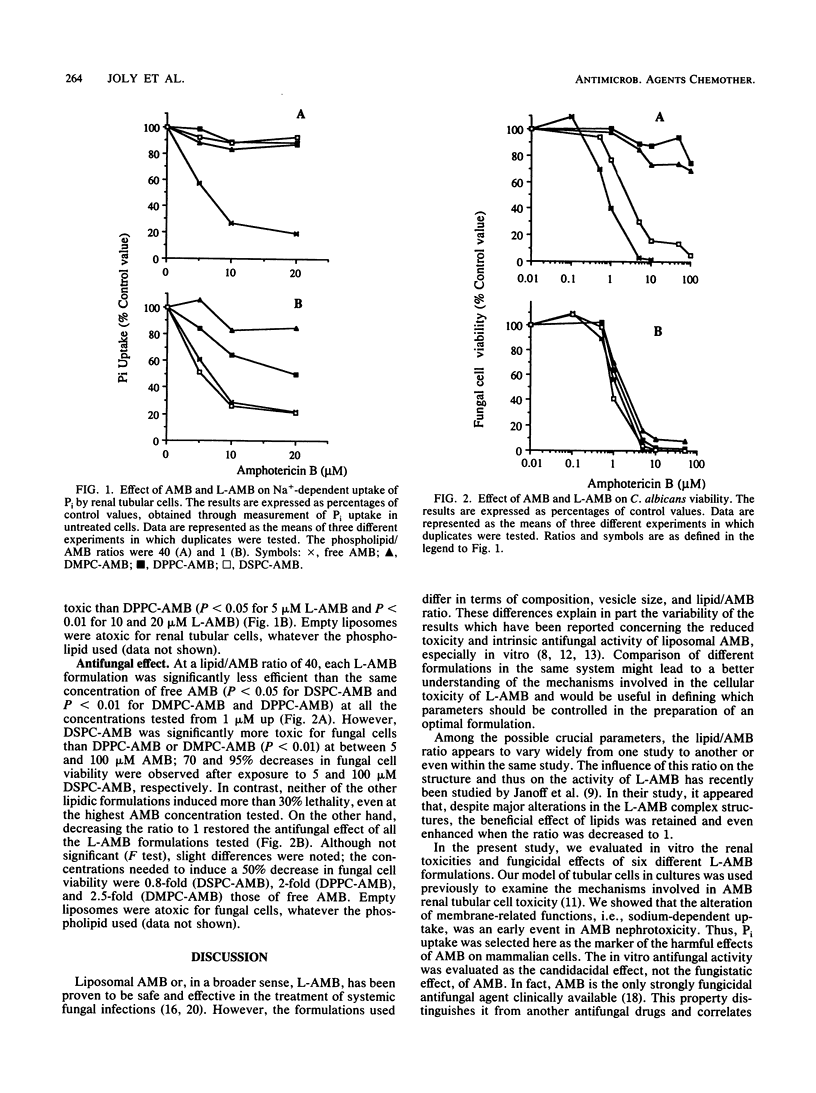
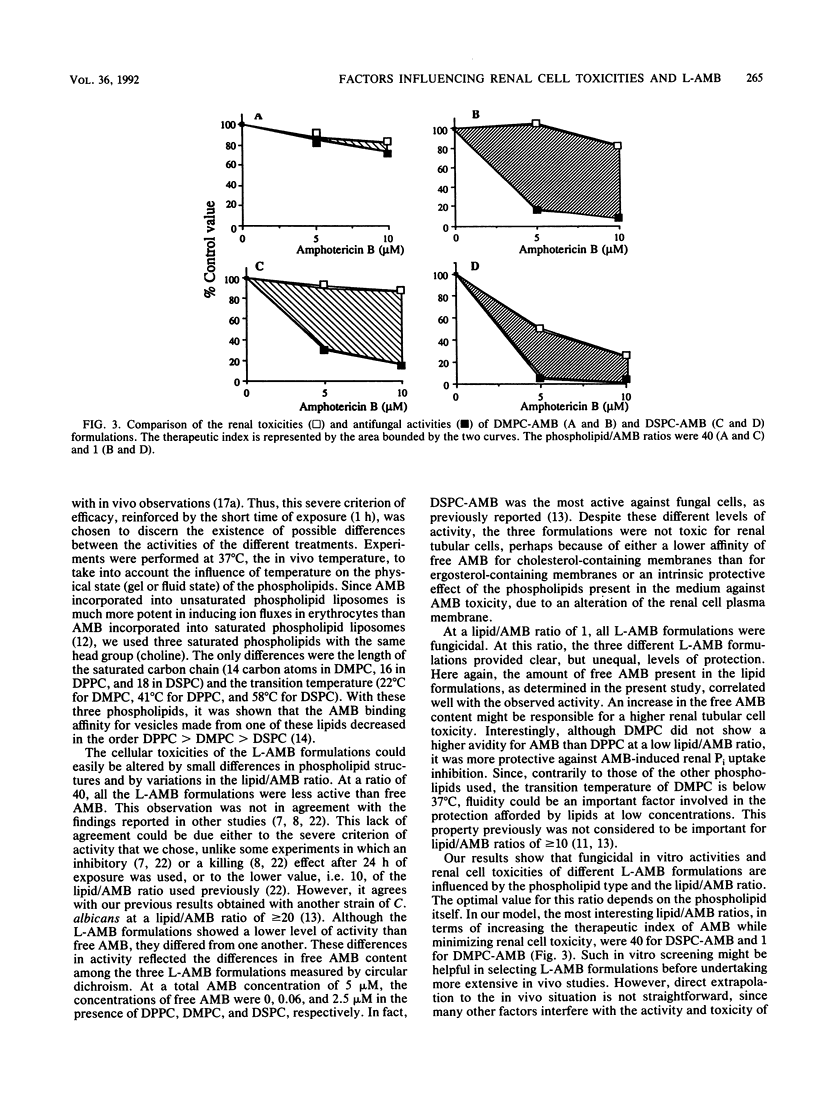
We studied the influence of the lipid/amphotericin B (AMB) ratio and the phospholipid type on the in vitro renal cell toxicity and antifungal efficacy of lipid-associated AMB (L-AMB). L-AMB was prepared at one of two different lipid/AMB ratios (1 and 40) by incubating AMB with empty small unilamellar vesicles, made from one of three different phospholipids: dipalmitoyl-, dimirystoyl-, and distearoylphosphatidylcholine (DPPC, DMPC, and DSPC, respectively). Renal cell toxicity, investigated through an assessment of the Na-dependent uptake of phosphate by proximal tubular cells, and fungicidal effect against Candida albicans were studied after 1 h of treatment at 37 degrees C. The amount of unbound AMB present in each L-AMB formulation was studied by use of circular dichroism. At a lipid/AMB ratio of 40, the three lipidic formulations were not toxic for renal cells but were less effective against C. albicans than AMB; however, DSPC-AMB, which contained 50% unbound AMB, was more effective against C. albicans than DPCC-AMB or DMPC-AMB, containing 0 and 13% unbound AMB, respectively. At a lipid/AMB ratio of 1, the antifungal effects of L-AMB and AMB were similar, whatever the phospholipid used, but only DMPC-AMB remained highly protective against AMB renal cell toxicity, despite the presence of the same amount of unbound AMB (50%) in DMPC-AMB and DPPC-AMB. We conclude that the in vitro activities and renal cell toxicities of different L-AMB formulations are influenced by the phospholipid type and the lipid/AMB ratio. The optimal ratio depends on the phospholipid itself. At a lipid/AMB ratio of 40, the antifungal activity depends mainly on the amount of unbound AMB in the formulation. At a lipid/AMB ratio of 1, the renal cell toxicity also depends on the fluidity of the phospholipid.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986 Dec 22;864(3-4):257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brajtburg J., Powderly W. G., Kobayashi G. S., Medoff G. Amphotericin B: current understanding of mechanisms of action. Antimicrob Agents Chemother. 1990 Feb;34(2):183–188. doi: 10.1128/aac.34.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander G., Amiel C. Protein kinase C activation has dissimilar effects on sodium-coupled uptakes in renal proximal tubular cells in primary culture. J Biol Chem. 1989 Mar 5;264(7):3935–3941. [PubMed] [Google Scholar]
- Holleran W. M., Wilbur J. R., DeGregorio M. W. Empiric amphotericin B therapy in patients with acute leukemia. Rev Infect Dis. 1985 Sep-Oct;7(5):619–624. doi: 10.1093/clinids/7.5.619. [DOI] [PubMed] [Google Scholar]
- Hopfer R. L., Mehta R., Lopez-Berestein G. Synergistic antifungal activity and reduced toxicity of liposomal amphotericin B combined with gramicidin S or NF. Antimicrob Agents Chemother. 1987 Dec;31(12):1978–1981. doi: 10.1128/aac.31.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopfer R. L., Mills K., Mehta R., Lopez-Berestein G., Fainstein V., Juliano R. L. In vitro antifungal activities of amphotericin B and liposome-encapsulated amphotericin B. Antimicrob Agents Chemother. 1984 Mar;25(3):387–389. doi: 10.1128/aac.25.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoff A. S., Boni L. T., Popescu M. C., Minchey S. R., Cullis P. R., Madden T. D., Taraschi T., Gruner S. M., Shyamsunder E., Tate M. W. Unusual lipid structures selectively reduce the toxicity of amphotericin B. Proc Natl Acad Sci U S A. 1988 Aug;85(16):6122–6126. doi: 10.1073/pnas.85.16.6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly V., Dromer F., Barge J., Yeni P., Seta N., Molas G., Carbon C. Incorporation of amphotericin B (AMB) into liposomes alters AMB-induced acute nephrotoxicity in rabbits. J Pharmacol Exp Ther. 1989 Oct;251(1):311–316. [PubMed] [Google Scholar]
- Joly V., Saint-Julien L., Carbon C., Yeni P. Interactions of free and liposomal amphotericin B with renal proximal tubular cells in primary culture. J Pharmacol Exp Ther. 1990 Oct;255(1):17–22. [PubMed] [Google Scholar]
- Juliano R. L., Grant C. W., Barber K. R., Kalp M. A. Mechanism of the selective toxicity of amphotericin B incorporated into liposomes. Mol Pharmacol. 1987 Jan;31(1):1–11. [PubMed] [Google Scholar]
- Jullien S., Contrepois A., Sligh J. E., Domart Y., Yeni P., Brajtburg J., Medoff G., Bolard J. Study of the effects of liposomal amphotericin B on Candida albicans, Cryptococcus neoformans, and erythrocytes by using small unilamellar vesicles prepared from saturated phospholipids. Antimicrob Agents Chemother. 1989 Mar;33(3):345–349. doi: 10.1128/aac.33.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullien S., Vertut-Croquin A., Brajtburg J., Bolard J. Circular dichroism for the determination of amphotericin B binding to liposomes. Anal Biochem. 1988 Jul;172(1):197–202. doi: 10.1016/0003-2697(88)90432-0. [DOI] [PubMed] [Google Scholar]
- Krause H. J., Juliano R. L. Interactions of liposome-incorporated amphotericin B with kidney epithelial cell cultures. Mol Pharmacol. 1988 Sep;34(3):286–297. [PubMed] [Google Scholar]
- Lopez-Berestein G., Bodey G. P., Frankel L. S., Mehta K. Treatment of hepatosplenic candidiasis with liposomal-amphotericin B. J Clin Oncol. 1987 Feb;5(2):310–317. doi: 10.1200/JCO.1987.5.2.310. [DOI] [PubMed] [Google Scholar]
- Lopez-Berestein G., Mehta R., Hopfer R. L., Mills K., Kasi L., Mehta K., Fainstein V., Luna M., Hersh E. M., Juliano R. Treatment and prophylaxis of disseminated infection due to Candida albicans in mice with liposome-encapsulated amphotericin B. J Infect Dis. 1983 May;147(5):939–945. doi: 10.1093/infdis/147.5.939. [DOI] [PubMed] [Google Scholar]
- Medoff G., Brajtburg J., Kobayashi G. S., Bolard J. Antifungal agents useful in therapy of systemic fungal infections. Annu Rev Pharmacol Toxicol. 1983;23:303–330. doi: 10.1146/annurev.pa.23.040183.001511. [DOI] [PubMed] [Google Scholar]
- Pisarik L., Joly V., Jullien S., Carbon C., Yeni P. Reduction of free amphotericin B acute toxicity in mice after intravenous administration of empty liposomes. J Infect Dis. 1990 May;161(5):1042–1044. doi: 10.1093/infdis/161.5.1042. [DOI] [PubMed] [Google Scholar]
- Sculier J. P., Coune A., Meunier F., Brassinne C., Laduron C., Hollaert C., Collette N., Heymans C., Klastersky J. Pilot study of amphotericin B entrapped in sonicated liposomes in cancer patients with fungal infections. Eur J Cancer Clin Oncol. 1988 Mar;24(3):527–538. doi: 10.1016/s0277-5379(98)90033-5. [DOI] [PubMed] [Google Scholar]
- Stamm A. M., Diasio R. B., Dismukes W. E., Shadomy S., Cloud G. A., Bowles C. A., Karam G. H., Espinel-Ingroff A. Toxicity of amphotericin B plus flucytosine in 194 patients with cryptococcal meningitis. Am J Med. 1987 Aug;83(2):236–242. doi: 10.1016/0002-9343(87)90691-7. [DOI] [PubMed] [Google Scholar]
- Szoka F. C., Jr, Milholland D., Barza M. Effect of lipid composition and liposome size on toxicity and in vitro fungicidal activity of liposome-intercalated amphotericin B. Antimicrob Agents Chemother. 1987 Mar;31(3):421–429. doi: 10.1128/aac.31.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor R. L., Williams D. M., Craven P. C., Graybill J. R., Drutz D. J., Magee W. E. Amphotericin B in liposomes: a novel therapy for histoplasmosis. Am Rev Respir Dis. 1982 May;125(5):610–611. doi: 10.1164/arrd.1982.125.5.610. [DOI] [PubMed] [Google Scholar]
- Tremblay C., Barza M., Fiore C., Szoka F. Efficacy of liposome-intercalated amphotericin B in the treatment of systemic candidiasis in mice. Antimicrob Agents Chemother. 1984 Aug;26(2):170–173. doi: 10.1128/aac.26.2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vertut-Croquin A., Bolard J., Chabbert M., Gary-Bobo C. Differences in the interaction of the polyene antibiotic amphotericin B with cholesterol- or ergosterol-containing phospholipid vesicles. A circular dichroism and permeability study. Biochemistry. 1983 Jun 7;22(12):2939–2944. doi: 10.1021/bi00281a024. [DOI] [PubMed] [Google Scholar]



