Abstract
Myocardial fibrosis caused by maladaptive extracellular matrix (ECM) remodeling is implicated in the dysfunction of the failing heart. Matrix metalloproteinases (MMPs) regulate ECM remodeling, and are regulated by cytokines. Transgenic mice with cardiac-specific overexpression of tumor necrosis factor α (TNF-α) (TNF1.6) develop heart failure. We hypothesized that modulation of TNF-α and/or MMP activity might alter the myocardial ECM remodeling process and the development of heart failure. To test this hypothesis, we took advantage of the TNF1.6 mice and studied soluble and total collagens and collagen type profiling by using hydroxyproline quantification, Sircol collagen assay, Northern blot analysis, and immunohistochemistry and studied myocardial function by using echocardiography. Progressive ventricular hypertrophy and dilation in the TNF1.6 mice were accompanied by a significant increase in MMP-2 and MMP-9 activity, an increase in collagen synthesis, deposition, and denaturation, and a decrease in undenatured collagens. In young TNF1.6 mice, these changes in the ECM were associated with marked diastolic dysfunction as demonstrated by significantly reduced transmitral Doppler echocardiographic E/A wave ratio. Anti-TNF-α treatment with adenoviral vector expressing soluble TNF-α receptor type I attenuated both MMP-2 and MMP-9 activity, prevented further collagen synthesis, deposition and denaturation, and preserved myocardial diastolic function in young, but not old, TNF1.6 mice. The results suggest a critical role of TNF-α and MMPs in myocardial matrix remodeling and functional regulation and support the hypothesis that both TNF-α and MMPs may serve as potential therapeutic targets in the treatment of heart failure.
Myocardial fibrosis and maladaptive extracellular matrix (ECM) remodeling are pathognomonic findings in end-stage congestive failing heart (1). Collagen fibers form the scaffolding of the myocardium and are required for the development and distribution of myocardial contractile force (2). Several distinct types of collagen can be found in the adult heart with type I collagen being predominant (3). Changes in myocardial total collagen content (4), collagen subtypes (5), and collagen denaturation as well as collagen cross-linking (6, 7) are important features of ECM remodeling and contribute to the myocardial stiffness and diastolic and systolic dysfunction seen in both animal models and patients with heart failure and may have prognostic significance. We hypothesized that modulation of myocardial matrix metabolism through regulation of upstream instigators of ECM remodeling could provide further insights for therapies of heart failure.
Recent evidence suggests that altered matrix metalloproteinases (MMPs, EC 3.4.24) may play an important role in cardiac ECM remodeling (8, 9). MMPs are a family of functionally related enzymes that cleave matrix components, resulting in fibrillar collagen denaturation and degradation, and the synthesis of new fibrotic tissue (10–12). That MMPs play an important role in regulating ECM turnover and in affecting myocardial geometry is seen by the finding that enhanced MMP activation found in patients with heart failure is associated with progressive left ventricle (LV) dilation and sphericalization (13, 14). Furthermore, MMP inhibition has been shown to limit ECM destruction and improve myocardial structure and function in animal models (15).
The expression of MMPs can be regulated at both pre- and posttranscriptional levels, and the activity of MMPs can be attenuated by physiologic inhibitors (10, 16). However, the signaling pathway responsible for regulating MMP expression and function have not been well defined. Recent studies suggest that MMPs are increased in response to tumor necrosis factor α (TNF-α; refs. 17–20). Furthermore, transgenic mice with cardiac specific overexpression of TNF-α (TNF1.6) demonstrate the characteristic heart failure phenotype: reduced adrenergic responsiveness, interstitial fibrosis, and LV dilation (21). These findings led us to hypothesize that cardiac re-expression of TNF-α may be responsible for increased MMPs, thus leading to ECM remodeling and myocardial dysfunction. In addition, we hypothesized that inhibition of TNF-α bioactivity might attenuate MMPs and matrix remodeling within the failing heart. To test these hypotheses, we assessed the activity of MMPs, collagen expression, deposition and denaturation, and myocardial function during the development of heart failure in TNF1.6 mice. We also investigated the effects of anti-TNF-α therapy by using adenovirus-mediated gene transfer of recombinant soluble TNF-α receptor type I (AdTNFRI) on myocardial ECM remodeling and function in both young and old TNF1.6 mice.
Materials and Methods
TNF-α Transgenic Mice and Cardiac Sample Collection.
TNF1.6 mice were created by microinjection of murine TNF-α transgene constructs into the pronuclei of one-cell FVB mouse embryos, after which the embryos were surgically implanted into pseudopregnant mice. Founders harboring the TNF-α transgene were identified by PCR and used for breeding. The offspring TNF1.6 mice develop cardiac hypertrophy, dilation, and failure (21).
TNF1.6 and FVB control mice were euthanized by methoxy flurane overdose, and ventricle and body weights were recorded. LV tissues were divided for histological analysis (fixed in 4% paraformaldehyde) and for protein and enzymatic analyses (snap-frozen in liquid nitrogen). This study was performed according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Measurement of MMP Activity.
MMP activity in 100 μg of myocardial extracts was measured by gelatin zymography exactly as described (9, 22). The gelatinolytic zones were quantified by using imagequant software (Molecular Dynamics).
Collagen Expression and Content Determination.
The transcript levels of both proα1(I) and proα1(III) for type I and type III collagens were determined by Northern blot analysis using mouse proα1(I) and proα1(III) cDNA probes (23).
Total soluble collagens were extracted overnight by using 5 mg/ml pepsin in 0.5 M acetic acid (24). Collagen contents were determined by hydroxyproline quantification using both hydroxyproline and type I collagen as standards (25–27). The soluble collagens included both denatured and undenatured ones with the latter measurable by the Sircol collagen assay (Accurate Chemicals). Denatured collagens lose the triple-helix structure and the ability to bind picrosirius red because of excessive exposure to MMPs.
Collagen immunohistochemistry was performed with antibodies against either mouse type I collagen (Chemicon) or human type III collagen (Biodesign International, Kennebunkport, ME). Sections were observed by using a fluorescence microscope, and the identity of the samples was blinded to the examiner. Five images were taken from each section by using the optimas image analysis software (Media Cybernetics, Silver Spring, MD) and a Sony video camera connected to the microscope. The collagen volume fraction was calculated as the area occupied by collagens divided by the total area in the visual field (28, 29).
Adenoviral-Soluble TNF Receptor Type I Vector Treatment.
AdTNFRI encodes a fusion protein containing the soluble TNF-α receptor type I fused with murine IgG (30). AdLacZ encodes Escherichia coli β-galactosidase. Both young (6 weeks old) and old (42 weeks old) TNF1.6 mice were injected in the retro-orbital veinal plexus with 109 plaque-forming units of AdTNFRI or AdLacZ and studied after 6 weeks (30, 31). The young mice also were treated for 2 weeks. The final ages of each treatment group were 8 weeks, 12 weeks, and 48 weeks, respectively.
Echocardiography.
TNF1.6 and FVB wild-type mice were anesthetized with i.p. injection of 2.5% Avertin (18 μg/g body weight). Echocardiography was performed with a Sequoia 512 Ultrasonograph (Acuson, Mountain View, CA). A 13-MHz linear transducer was placed on a layer of acoustic coupling gel applied to the left hemithorax. Diastolic transmitral LV inflow velocities were interrogated from angulated parasternal long-axis views by using a Doppler transducer (7 MHz) with a sample volume length of 3.5–7.5 mm. Two-dimensionally targeted M-mode and color flow mapping-guided pulsed-wave Doppler studies were performed at baseline and after i.p. administration of isoproterenol (300 ng/g body weight).
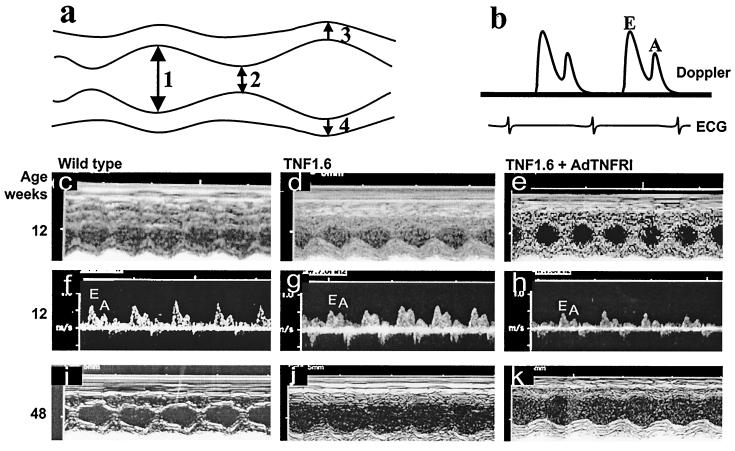
M-mode measurements of LV end diastolic and end systolic diameters were measured (see Fig. 6a) and used for the calculation of fractional shortening of the LV. End diastole was determined at the maximal LV diastolic dimension, and end systole was taken at the peak of posterior wall motion. Three beats were averaged for each measurement. Spectral Doppler waveforms were analyzed for peak early- and late-diastolic transmitral velocities. The E/A wave ratio (peak early- to late-transmitral flow velocity ratio) was calculated as described (32).
Figure 6.
Echocardiographic measurement of the geometry and function of the mouse heart. LV dimensions were calculated from measurements of end-diastolic diameter (arrow 1), end-systolic diameter (arrow 2), anterior wall (arrow 3), and posterior wall (arrow 4), and E/A wave ratio was calculated as shown in schematic M-mode (a) and Doppler (b) echocardiographic tracings. Ventricular hypertrophy in 12-week-old TNF1.6 mice (d), ventricular dilation in 48-week-old TNF1.6 mice (j) as compared with respective wild-type mice (c and i). AdTNFRI treatment had no effect on ventricular hypertrophy or dilation (e and k), but the reduced E/A ratio in 12-week-old TNF1.6 mice (g) was prevented by the treatment (h).
Statistical Analysis.
Independent t test or one-way ANOVA was applied to compare changes in different groups. Comparison among the means was performed with the post hoc Student-Newman-Kuels analysis test using the spss statistical analysis software (33). The quantitative data are presented as mean ± SE unless specified otherwise. Statistical significance was considered at P < 0.05.
Results
Increased Myocardial MMPs and Suppression by Anti-TNF-α Therapy.
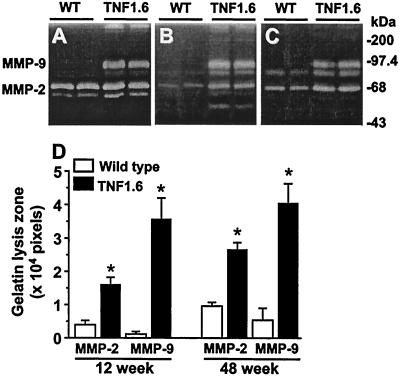
The activity of MMPs as measured by gelatin zymography was increased in the myocardium of neonatal, 12- and 48-week-old TNF1.6 mice when compared with age-matched wild-type controls (Fig. 1). MMP-9 (EC 3.4.24.35) activity was hardly detectable in wild-type mice, but was prominent in TNF1.6 mouse heart. MMP-2 (EC 3.4.24.24) activity was increased by more than 2-fold in both 12- and 48-week-old TNF1.6 mice (P < 0.015).
Figure 1.
Gelatin zymography of ventricular extracts of neonates (A), 12-week-old (B), 48-week-old (C) wild-type (WT) and TNF1.6 mice. (D) Summary of quantitative data of MMP-2 and MMP-9 activity in 12- and 48-week-old mouse heart (n = 4 each). *, P < 0.01 compared with respective wild type.
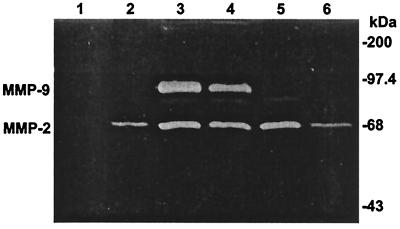
AdTNFRI treatment begun at 6 weeks of age abolished the activity of MMP-9 after 2 weeks. By contrast, the activity of MMP-2 was reduced to wild-type level only after 6 weeks of treatment (Fig. 2). Thus, in the remainder of our experiments we studied the 6-week treatment time point.
Figure 2.
Modulation of the activity of MMPs in the TNF1.6 mouse heart. Gelatin zymography demonstrates that AdTNFRI treatment abolished MMP-9 gelatinolytic activity after 2 weeks (lane 5) and reduced MMP-2 activity to wild-type level after 6 weeks (lane 6). Controls are negative without known MMPs (lane 1), FVB wild type (lane 2), TNF1.6 (lane 3), and TNF1.6 treated with AdLacZ control vector (lane 4).
ECM Remodeling and Its Modulation by Anti-TNF-α Therapy.
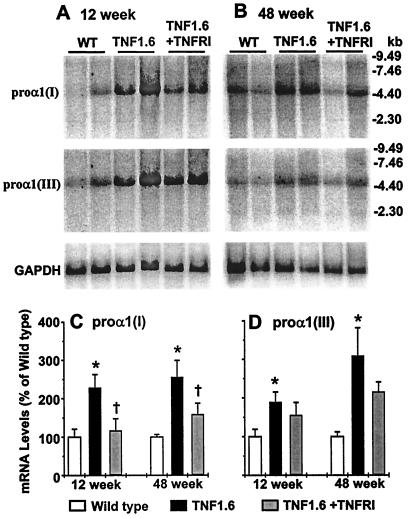
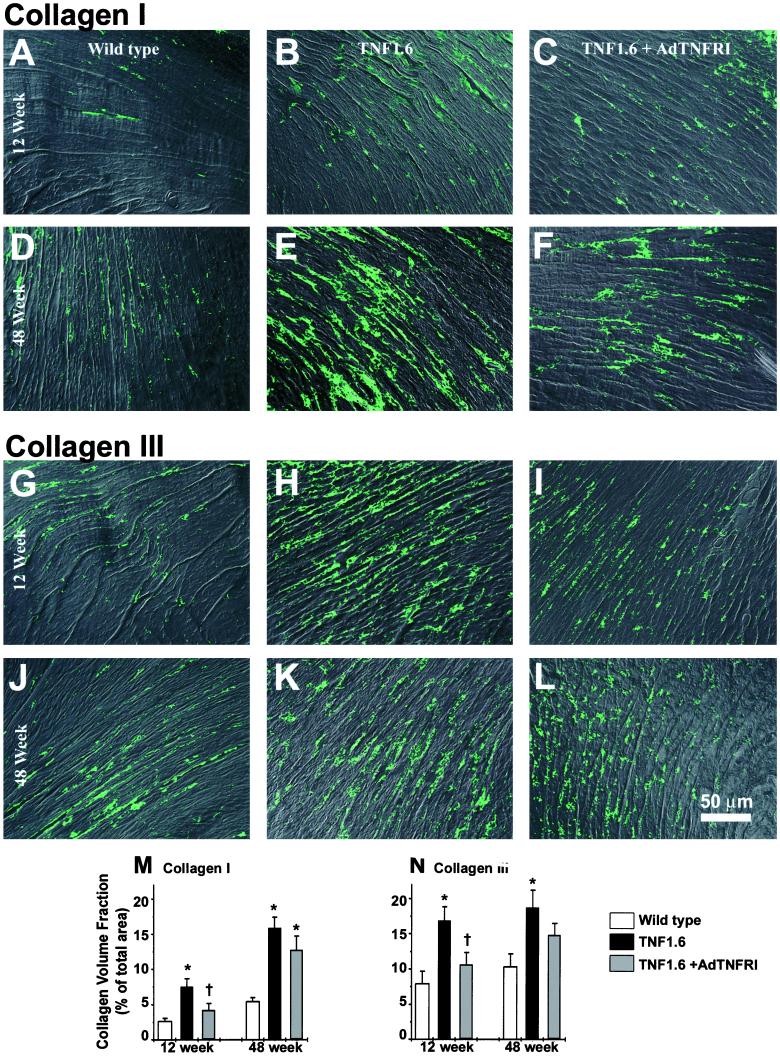
Collagens, the major components of fibrotic tissue, are the principal substrate of MMPs. The sum balance of collagen deposition is a function of both synthesis and degradation. To assess the contribution of increased collagen gene expression to fibrosis, and the regulation of collagen gene expression by AdTNFRI treatment, Northern blot analyses were performed. Relative to wild-type controls, both proα1(I) and proα1(III) collagen transcripts were significantly elevated in 12- and 48-week-old TNF1.6 mouse hearts, with proα1(I) reduced after AdTNFRI treatment (Fig. 3). Increased collagen transcripts were reflected in changes at the protein level in that both type I and III collagens were increased in TNF1.6 mouse heart as demonstrated by immunohistochemistry (Fig. 4). Hydroxyproline quantification also demonstrated an increase in both total collagens and total soluble collagens in the TNF1.6 mouse heart, and the increase was prevented by AdTNFRI treatment in 12-week-old mice. However, in older animals the same treatment failed to prevent further collagen deposition (Fig. 5C).
Figure 3.
Representative Northern blot analysis results of collagen proα1(I) and proα1(III). The same blot was sequentially hybridized with proα1(I), proα1(III), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) probes. The bar graphs summarize the quantitative results of proα1(I) and proα1(III) mRNA levels normalized to that of GAPDH in both 12- and 48-week-old wild-type (WT), TNF1.6, and TNF1.6 mice treated with AdTNFRI (n = 4 each). *, P < 0.05 compared with wild type; †, P < 0.05 compared with TNF1.6.
Figure 4.
(On the opposite page.) Indirect immunofluorescence of myocardial type I and type III collagens. (A–F) Type I collagen. (G–L) Type III collagen. (A–C and G–I) 12-week-old mice; (D–F and J–L) 48-week-old mice. Both type I and type III collagens appear increased in TNF-α transgenic (B, E, H, and K) relative to wild-type mice (A, D, G, and J), which were prevented by AdTNFRI treatment in 12-week-old mice (C and I) but not in 48-week-old mice (F and L). Original magnification: ×200. Quantified collagen volume fraction shows that the increased collagens I and III were prevented by AdTNFRI treatment in 12-week-old mice (n = 3) (M and N). *, P < 0.05 compared with wild type; †, P < 0.05 compared with TNF1.6.
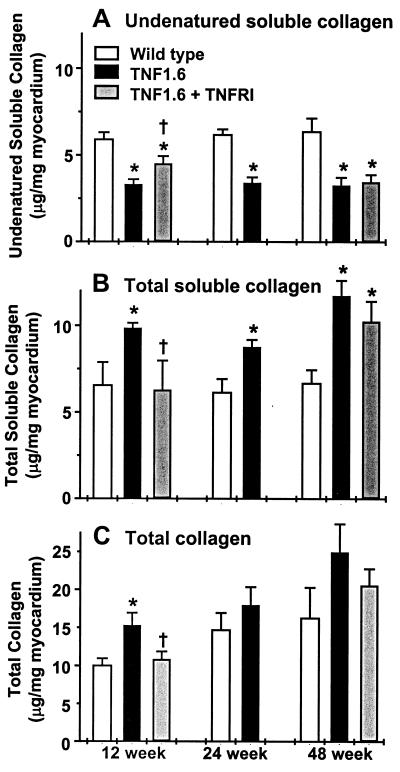
Figure 5.
Changes in collagens in the mouse heart. (A) Reduction in undenatured collagen in TNF1.6 mice was partially prevented by AdTNFRI treatment. (B) Total soluble collagen content was increased in 12-, 24-, and 48-week-old TNF1.6 mouse heart, and the increase was prevented by AdTNFRI treatment in 12-week-old TNF1.6 mice. (C) Gradual increase in total collagen content was seen in both wild-type and TNF1.6 mice, but was more apparent in TNF1.6 mice. The collagen accumulation in 12-week-old TNF1.6 mouse heart was prevented by AdTNFRI treatment. n = 8 in each group. *, P < 0.05 compared with wild type; †, P < 0.05 compared with TNF1.6.
The total soluble collagen can be further divided into undenatured soluble collagen, measurable by the Sircol collagen assay, and denatured soluble collagen, which is nonreactive with the Sircol collagen assay. Denatured soluble collagen can be generated by MMP activity. As shown in Fig. 5, whereas total soluble collagen contents were increased at all ages in TNF1.6 mice relative to wild-type controls, the undenatured soluble collagen content was decreased, suggesting increased content of denatured soluble collagen and increased MMP activity, consistent with the results for measurements of MMP activity (Fig. 1). Furthermore, AdTNFRI therapy significantly increased the undenatured soluble collagen content in young, but not in old, TNF1.6 mouse hearts (Fig. 5A).
Anti-TNF-α Therapy Improved Myocardial Diastolic Function.
Changes in collagen properties have been associated with alterations in myocardial diastolic function (4, 5, 7). Thus, the increase in collagen content and denaturation in the TNF1.6 mouse heart could be expected to cause stiffness whereas AdTNFRI treatment would be expected to improve diastolic function. As seen in Fig. 6 and Table 1, the TNF1.6 mice developed cardiac hypertrophy by 12 weeks of age (Fig. 6d) and marked ventricular dilation by 48 weeks (Fig. 6j). When cardiac systolic performance was assessed by LV fractional shortening, and diastolic function by the E/A ratio by echocardiography, 12-week-old transgenic mice did not demonstrate abnormalities in systolic performance at baseline or in response to isoproterenol; however, the E/A ratio was significantly reduced in TNF1.6 mice relative to wild-type mice (P < 0.05), suggesting increased LV stiffness and diastolic dysfunction (34). By 48 weeks of age there were marked abnormalities in baseline fractional shortening, as well as in response to isoproterenol (Table 1). However, measurements of E/A ratio could not be performed in 48-week-old mice because of atrial-ventricular conduction abnormalities and extreme chamber dilation.
Table 1.
Measurements of mouse myocardial structure and function
| Age, weeks | Measurement | Wild type, mean ± SD, n = 4 | TNF1.6, mean ± SD, n = 6 | TNF1.6 + AdTNFRI, mean ± SD, n = 6 |
|---|---|---|---|---|
| 12 | VW/BW | 3.92 ± 0.3 | 5.03 ± 0.41* | 5.19 ± 0.39* |
| LVED | 3.72 ± 0.26 | 4.05 ± 0.19 | 3.8 ± 0.14 | |
| FS (%) | 37.11 ± 2.41 | 35.64 ± 3.00 | 38.97 ± 2.77 | |
| FS (%) + Iso | 59.53 ± 4.68† | 52.27 ± 15.19† | 54.25 ± 7.92† | |
| E/A ratio | 1.71 ± 0.30 | 1.26 ± 0.16* | 1.58 ± 0.27‡ | |
| 48 | VW/BW | 3.49 ± 0.12 | 4.86 ± 0.22* | 4.62 ± 0.57* |
| LVED | 3.79 ± 0.17 | 4.63 ± 0.59* | 4.27 ± 0.31* | |
| FS (%) | 40.18 ± 7.81 | 27.1 ± 6.50* | 28.91 ± 2.53* | |
| FS (%) + Iso | 65.7 ± 15.7† | 29.82 ± 2.35 | 37.55 ± 3.78†‡ |
VW, ventricular weight (mg); BW, body weight (g); LVED, left ventricular end diastolic diameter (mm); FS, fractional shortening; Iso, isoproterenol.
, P < 0.05 vs. wild type;
, P < 0.01 vs. Iso unchallenged;
, P < 0.05 vs. TNF1.6.
That changes in collagen content and denaturation other than myocardial hypertrophy contribute to myocardial diastolic dysfunction was further demonstrated by treatment of TNF1.6 mice with AdTNFRI. The treatment did not change myocardial hypertrophy (Table 1), but significantly reduced collagen transcripts (Fig. 3), total collagen content, and collagen denaturation (Figs. 4 and 5). These changes were associated with significantly improved E/A ratio in 12-week-old TNF1.6 mice (Fig. 6 and Table 1, P < 0.05).
Discussion
Previous studies from our laboratory and by others have demonstrated that TNF-α modulates the expression of MMPs in vitro (17, 35). In the present study we demonstrate that transgenic overexpression of TNF-α effects a robust increase in MMP-2 and MMP-9 activity in vivo, a change that could be demonstrated from the initial postnatal period. Increased MMPs have been previously found in the failing hearts of both animal models and humans (8, 9, 15, 36). Several different mechanisms might account for the marked increase in MMPs in the TNF1.6 mouse heart: (i) increased stimulators of MMP-producing cells including macrophage factors, lymphokines, and soluble collagen fragments; (ii) increased cellular infiltrates; (iii) increased activators of MMPs including locally produced plasmin; and (iv) increased MMP production by cardiac cells. Interstitial infiltrates might have accounted for increased MMP-9 as the infiltration was increased and MMP-9 activity was elevated in white blood cells of TNF1.6 mice. However, infiltrating cells could not explain the marked increase in MMP-2 activity as no MMP-2 was detectable in white blood cells (see supplemental text and Figs. 7–9, which are published on the PNAS website, www.pnas.org).
Theoretically, an increase in MMP activity would result in a decrease in the MMP substrate: collagens. However, the contrary is usually true, i.e., an increase in MMP activity is accompanied by increased fibrosis whereas inhibition of MMP activity is associated with less deposition of fibrotic tissue (37, 38). This paradox is due to the fact that the total matrix collagen content is a function of both synthesis and degradation, and degraded products of matrix proteins (matrikines) may serve as stimulators for collagen synthesis (11, 12, 39), which may in turn result in increased deposition of poorly structured fibrotic tissue in the myocardium. Not surprisingly, in the TNF1.6 mice the increase in MMP activity was associated with an increase in the concentration of matrix collagens.
Although the difference in collagen contents between wild-type and TNF1.6 mice was more obvious in 12-week-old mice than in older TNF1.6 mice, the quality of collagens was reduced in both groups. Both total collagens and total soluble collagens were significantly increased, whereas undenatured soluble collagens were significantly reduced in TNF1.6 mouse heart. The results suggest that increased MMPs in the TNF1.6 mouse heart damaged the normal collagenous matrix. Thus, it is not surprising that these changes in collagen properties were associated with the development of progressive failure in this mouse model.
That changes in matrix collagen quantity and quality were of physiologic significance was demonstrated by the fact that diastolic function, as measured echocardiographically by the E/A ratio, was abnormal in the TNF1.6 transgenic mice. Prevention of changes in collagens by AdTNFRI therapy significantly improved diastolic function in the TNF1.6 mouse. Treatment of TNF1.6 mice with AdTNFRI has been shown to result in high levels of soluble TNFRI in plasma as well as in the myocardium and abrogates the myocardial inflammation (31). Six weeks of therapy with AdTNFRI in young TNF1.6 mice not only reduced MMP-2 and MMP-9 activity, but also effectively reduced collagen transcription, collagen content, and denaturation (Figs. 2–5). These changes were associated with marked improvement in diastolic function and supported the hypothesis that TNF-α regulates ECM remodeling in vivo through the regulation of MMPs. These results may be useful in interpreting the results of ongoing clinical trials assessing the efficiency of anti-TNF-α therapy with soluble TNF-α receptor in patients with chronic heart failure. Furthermore, it will be important to use techniques directly targeted at the MMPs to better understand their role in ECM remodeling and their potential as therapeutic targets in patients with heart failure.
In summary, TNF1.6 mice develop ventricular hypertrophy and dilation that are accompanied by significant increases in MMP-2 and MMP-9, collagen deposition, and collagen denaturation. In young mice, these changes are associated with marked diastolic dysfunction. Anti-TNF-α treatment attenuated MMPs, prevented further collagen deposition and denaturation, and improved myocardial diastolic function. These results suggest a critical role of TNF-α and MMP in the remodeling of myocardial matrix and regulation of myocardial function, and both TNF-α and MMPs may serve as a target for the treatment of heart failure.
Supplementary Material
Acknowledgments
We thank George Bounoutas for preparation and administration of AdTNFRI to the mice. This work was supported in part by National Institutes of Health Grant HL60032–01 and American Heart Association Grant 9804726U.
Abbreviations
- TNF-α
tumor necrosis factor α
- ECM
extracellular matrix
- MMP
matrix metalloproteinase
- LV
left ventricle
- AdTNFRI
recombinant adenovirus encoding soluble TNF-α receptor I
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Weber K T, Brilla C G, Campbell S E, Zhou G, Matsubara L, Guarda E. Blood Pressure. 1992;1:75–85. doi: 10.3109/08037059209077497. [DOI] [PubMed] [Google Scholar]
- 2.Weber K T, Pick R, Jalil J E, Janicki J S, Carroll E P. J Mol Cell Cardiol. 1989;21, Suppl. 5:121–131. doi: 10.1016/0022-2828(89)90778-5. [DOI] [PubMed] [Google Scholar]
- 3.Weber K T. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 4.Jalil J E, Doering C W, Janicki J S, Pick R, Shroff S G, Weber K T. Circ Res. 1989;64:1041–1050. doi: 10.1161/01.res.64.6.1041. [DOI] [PubMed] [Google Scholar]
- 5.Mukherjee D, Sen S. Circ Res. 1990;67:1474–1480. doi: 10.1161/01.res.67.6.1474. [DOI] [PubMed] [Google Scholar]
- 6.Iimoto D S, Covell J W, Harper E. Circ Res. 1988;63:399–408. doi: 10.1161/01.res.63.2.399. [DOI] [PubMed] [Google Scholar]
- 7.Norton G R, Tsotetsi J, Trifunovic B, Hartford C, Candy G P, Woodiwiss A J. Circulation. 1997;96:1991–1998. doi: 10.1161/01.cir.96.6.1991. [DOI] [PubMed] [Google Scholar]
- 8.Thomas C V, Coker M L, Zellner J L, Handy J R, Crumbley A J, 3rd, Spinale F G. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- 9.Li Y Y, Feldman A M, Sun Y, McTiernan C F. Circulation. 1998;98:1728–1734. doi: 10.1161/01.cir.98.17.1728. [DOI] [PubMed] [Google Scholar]
- 10.Dollery C M, McEwan J R, Henney A M. Circ Res. 1995;77:863–868. doi: 10.1161/01.res.77.5.863. [DOI] [PubMed] [Google Scholar]
- 11.Maquart F X, Pickart L, Laurent M, Gillery P, Monboisse J C, Borel J P. FEBS Lett. 1988;238:343–346. doi: 10.1016/0014-5793(88)80509-x. [DOI] [PubMed] [Google Scholar]
- 12.Li Y Y, McTiernan C F, Feldman A M. Cardiovasc Res. 2000;46:214–224. doi: 10.1016/s0008-6363(00)00003-1. [DOI] [PubMed] [Google Scholar]
- 13.Janicki J S, Brower G L, Henegar J R, Wang L. Adv Exp Med Biol. 1995;382:239–245. doi: 10.1007/978-1-4615-1893-8_24. [DOI] [PubMed] [Google Scholar]
- 14.Gunja-Smith Z, Morales A R, Romanelli R, Woessner J F., Jr Am J Pathol. 1996;148:1639–1648. [PMC free article] [PubMed] [Google Scholar]
- 15.Spinale F G, Coker M L, Krombach S R, Mukherjee R, Hallak H, Houck W V, Clair M J, Kribbs S B, Johnson L L, Peterson J T, Zile M R. Circ Res. 1999;85:364–376. doi: 10.1161/01.res.85.4.364. [DOI] [PubMed] [Google Scholar]
- 16.Vincenti M P, White L A, Schroen D J, Benbow U, Brinckerhoff C E. Crit Rev Eukaryot Gene Exp. 1996;6:391–411. doi: 10.1615/critreveukargeneexpr.v6.i4.40. [DOI] [PubMed] [Google Scholar]
- 17.Li Y Y, McTiernan C F, Feldman A M. Cardiovasc Res. 1999;42:162–172. doi: 10.1016/s0008-6363(98)00297-1. [DOI] [PubMed] [Google Scholar]
- 18.Galis Z S, Muszynski M, Sukhova G K, Simon-Morrissey E, Libby P. Ann NY Acad Sci. 1995;748:501–507. doi: 10.1111/j.1749-6632.1994.tb17348.x. [DOI] [PubMed] [Google Scholar]
- 19.Lefebvre V, Peeters-Joris C, Vaes G. Biochim Biophys Acta. 1991;1094:8–18. doi: 10.1016/0167-4889(91)90020-x. [DOI] [PubMed] [Google Scholar]
- 20.Lee E, Vaughan D E, Parikh S H, Grodzinsky A J, Libby P, Lark M W, Lee R T. Circ Res. 1996;78:44–49. doi: 10.1161/01.res.78.1.44. [DOI] [PubMed] [Google Scholar]
- 21.Kubota T, McTiernan C F, Frye C S, Slawson S E, Lemster B H, Koretsky A P, Demetris A J, Feldman A M. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- 22.Tyagi S C, Matsubara L, Weber K T. Clin Biochem. 1993;26:191–198. doi: 10.1016/0009-9120(93)90025-2. [DOI] [PubMed] [Google Scholar]
- 23.Metsaranta M, Toman D, De Crombrugghe B, Vuorio E. Biochim Biophys Acta. 1991;1089:241–243. doi: 10.1016/0167-4781(91)90014-d. [DOI] [PubMed] [Google Scholar]
- 24.Miller E J, Rhodes R K. Methods Enzymol. 1982;82:33–64. doi: 10.1016/0076-6879(82)82059-4. [DOI] [PubMed] [Google Scholar]
- 25.Stegemann H, Stalder K. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 26.Mukherjee D, Sen S. J Clin Invest. 1991;88:1141–1146. doi: 10.1172/JCI115414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neuman R, Logan M. J Biol Chem. 1950;186:549–556. [PubMed] [Google Scholar]
- 28.Weber K T, Pick R, Silver M A, Moe G W, Janicki J S, Zucker I H, Armstrong P W. Circulation. 1990;82:1387–1401. doi: 10.1161/01.cir.82.4.1387. [DOI] [PubMed] [Google Scholar]
- 29.Shirani J, Pick R, Roberts W C, Maron B J. J Am Coll Cardiol. 2000;35:36–44. doi: 10.1016/s0735-1097(99)00492-1. [DOI] [PubMed] [Google Scholar]
- 30.Kolls J, Peppel K, Silva M, Beutler B. Proc Natl Acad Sci USA. 1994;91:215–219. doi: 10.1073/pnas.91.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kubota T, Bounoutas G, Miyagishima M, Kadokami T, Sanders V, Brudon C, Robbins P D, McTiernan C F, Feldman A M. Circulation. 2000;101:2518–2525. doi: 10.1161/01.cir.101.21.2518. [DOI] [PubMed] [Google Scholar]
- 32.Kovacs S J, Rosado J, Manson McGuire A L, Hall A F. Hypertension. 1997;30:788–795. doi: 10.1161/01.hyp.30.4.788. [DOI] [PubMed] [Google Scholar]
- 33.Wallenstein S, Zucker C L, Fleiss J L. Circ Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- 34.Litwin S E, Grossman W. J Am Coll Cardiol. 1993;22:49A–55A. doi: 10.1016/0735-1097(93)90463-b. [DOI] [PubMed] [Google Scholar]
- 35.Gum R, Wang H, Lengyel E, Juarez J, Boyd D. Oncogene. 1997;14:1481–1493. doi: 10.1038/sj.onc.1200973. [DOI] [PubMed] [Google Scholar]
- 36.Spinale F G, Coker M L, Thomas C V, Walker J D, Mukherjee R, Hebbar L. Circ Res. 1998;82:482–495. doi: 10.1161/01.res.82.4.482. [DOI] [PubMed] [Google Scholar]
- 37.Dixon I M C, Ju H S, Reid N L, Scammelllafleur T, Werner J P, Jasmin G. J Mol Cell Cardiol. 1997;29:1837–1850. doi: 10.1006/jmcc.1997.0420. [DOI] [PubMed] [Google Scholar]
- 38.Cowan K N, Jones P L, Rabinovitch M. Circ Res. 1999;84:1223–1233. doi: 10.1161/01.res.84.10.1223. [DOI] [PubMed] [Google Scholar]
- 39.Maquart F X, Bellon G, Chaqour B, Wegrowski J, Patt L M, Trachy R E, Monboisse J C, Chastang F, Birembaut P, Gillery P, et al. J Clin Invest. 1993;92:2368–2376. doi: 10.1172/JCI116842. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.