Abstract
Arterial inflammatory responses are thought to be a significant component of atherosclerotic disease. We describe here, using a transgenic approach, the mutual perpetuation of immune-mediated arterial inflammation and cholesterol-induced atherosclerosis. Mice expressing the bacterial transgene β-galactosidase exclusively in cardiomyocytes and in smooth muscle cells in lung arteries and the aorta (SM-LacZ), and hypercholesterolemic apolipoprotein E-deficient SM-LacZ mice (SM-LacZ/apoE−/−) developed myocarditis and arteritis after immunization with dendritic cells presenting a β-galactosidase-derived immunogenic peptide. Hypercholesterolemia amplified acute arteritis and perpetuated chronic arterial inflammation in SM-LacZ/apoE−/− mice, but had no major impact on acute myocarditis or the subsequent development of dilated cardiomyopathy. Conversely, arteritis significantly accelerated cholesterol-induced atherosclerosis. Taken together, these data demonstrate that the linkage of immune-mediated arteritis and hypercholesterolemia favors initiation and maintenance of atherosclerotic lesion formation. Therapeutic strategies to prevent or disrupt such self-perpetuating vicious circles may be crucial for the successful treatment of atherosclerosis.
Atherosclerosis is a chronic inflammatory disease developing in response to injury in the vessel wall. It is characterized by the infiltration of mononuclear lymphocytes into the intima, local expansion of vascular smooth muscle (SM) cells, and accumulation of extracellular matrix (1). Hypercholesterolemia has been identified as one of the most important risk factors for atherosclerosis in humans (2). In addition, the importance of hypercholesterolemia in atherogenesis has been well documented in animal models, including genetically modified mice lacking the low density lipoprotein (LDL) receptor (LDLR−/−) (3) or apolipoprotein E (apoE−/−) (4). The chronic inflammatory influence of hypercholesterolemia is thought to be mediated by induction of cytokines and chemokines (5), up-regulation of endothelial adhesion molecules (6), and immune reactions against oxidized moieties on lipoproteins (7, 8).
Initial vascular injury can result from cytopathic effects of infectious agents replicating in the vascular wall or can be mediated by an immune response directed against antigens present in SM or endothelial cells. In humans, herpesvirus DNA or antigen can be found in atherosclerotic lesions in patients with coronary artery disease (9, 10). In addition, there is a correlation between seroreactivity against Chlamydia pneumoniae and coronary heart disease (11), and C. pneunoniae antigen can be detected in patients with carotid artery stenosis (10).
Studies in the late 1970s demonstrated that infection of chickens with an avian herpesvirus can be associated with atherosclerotic lesions that resemble those found in humans (12). Nevertheless, it is difficult in virus-induced immunopathological disease to distinguish between direct virus-mediated tissue damage and the potentially disease-perpetuating inflammatory response. It is therefore helpful to uncouple microbial replication and antimicrobial immune responses to assess the role of immune-mediated vascular pathology. This may be achieved, for example, by using mycobacterial heat shock protein (hsp) for immunization of rabbits (13) or mice (14). However, immunization with mycobacterial hsp may also induce or aggravate other autoimmune diseases such as experimental arthritis (15) or diabetes (16), indicating that anti-hsp immune responses are not exclusively linked with vascular immunopathology. To overcome this obstacle, we used a transgenic mouse model with the defined expression of the microbial antigen β-galactosidase (β-gal) in arterial smooth muscle cells (SM-LacZ mice) (17). In SM-LacZ mice, strong anti-β-gal immune responses can be induced leading to a distinct immunopathology (18). By back-crossing these mice with hypercholesterolemic apoE−/− mice (4), we developed a transgenic model that allowed us to address the following questions: (i) What is the influence of hypercholesterolemia in the development of immune-mediated arterial inflammation? and (ii) What is the significance of immune-mediated arteritis in the chronological process of atherosclerosis as opposed to, or in combination with other cofactors, such as hypercholesterolemia?
Materials and Methods
Mice.
Mice were obtained from the Institut für Labortierkunde (University of Zurich) and maintained in the specific pathogen-free facilities of the University Hospital Zurich. Transgenic SM-LacZ mice expressing β-gal under the control of the SM22alpha-promotor (2126nlz) have been described (17). ApoE−/− mice (4), in a C57BL/6J background, were obtained from The Jackson Laboratories. SM-LacZ mice were crossed with apoE−/− mice, and heterozygous offspring were mated to produce apoE-deficient mice expressing β-gal in arterial SM cells. Mice were maintained on two different dietary regimens: (i) normal rodent chow (ND) for 14 days (no. 3430, Provimi Kliba, Kaiseraugst, Switzerland), or (ii) normal rodent chow for 14 days followed by 3 weeks of high-fat and high-cholesterol diet (ND supplement with 1.25% cholesterol, 8.75% fat, Provimi Kliba AG). Experiments were carried out with age- (5–6 wk) and sex-matched animals.
Preparation and Peptide Pulse of Dendritic Cells (DC).
Generation of DC from bone marrow cultures of C57BL/6 mice has been described (19). DC were pulsed with the H-2Kb-binding β-gal-peptide 497–504 (Neosystems Laboratoire, Strasbourg, France) at a concentration of 10-6 M for 60 min at 37°C.
Cytotoxicity Assay and Assessment of in Vivo T Helper Cell Activity.
Spleen cells (5 × 106/well) from primed mice were restimulated for 5 days in 24-well tissue culture plates with 3 × 106 β-gal 497–504-pulsed, irradiated (3,000 rad) spleen cells, and cytotoxicity was tested in a standard 5-h 51Cr-release assay as described (18).
Evaluation of Histopathological Alterations.
Freshly removed organs were immersed in Hanks' balanced salt solution and snap-frozen in liquid nitrogen. Frozen tissue sections were cut in a cryostat and fixed in acetone for 10 min. Sections were incubated with anti-mouse mAb against CD8+ cells (YTS169.4.2) (20) or against inflammatory macrophages (F4/80; HB-198, ATCC) followed by goat anti-rat Ig (Caltag, South San Francisco, CA) and alkaline phosphatase-labeled donkey anti-goat Ig (Jackson ImmunoResearch). Alkaline phophatase was visualized by using AS-BI phosphate/New Fuchsin, and sections were counterstained with hemalum. To assess the immunopathological alterations in the myocardium, lung arteries, and aortas after DC/β-gal 497–504 immunization a scoring system using the following parameters was established: (a) cellular infiltration with mononuclear cells (CD8 T cells and inflammatory macrophages) in the right ventricle wall, lung arteries, and aorta (score 0 for no infiltration; 1, <20% affected; 2, 20–40%; 3, 40–60%, 4, >60%); (b) quality of the infiltrate and loss of functional tissue in the myocardium (score 1 for <20% fibro-histiocytic infiltrate; 2, 20–40%; 3, >40%, no dilatation; 4, >40%, dilatation), lung arteries, and aorta (score 1 for mainly perivascular infiltrate; 2, single cells/small foci in the media; 3, large foci in media and intima, no occlusion of vessels; 4, occlusion of vessels). The range of histopathological scores was therefore from 0 (no alteration) to 8 (severe loss of functional tissue). Four to five heart sections, three lung sections, and 4–6 aortic arch sections from each mouse were evaluated. Sections were evaluated by two observers unfamiliar with the tested specimen. For the quantitative evaluation of atherosclerotic lesions, 3–4 serial cross-sections through the aortic origin, beginning with the appearance of all three valve cusps, were stained with Oil Red O, counterstained with hemalum, and measured by using a Zeiss Axioskop 50 microscope and axiohome computer-aided morphometry software (Zeiss). The average lesion size for each mouse was calculated, and statistical analysis was performed by the use of prism 2.01 software (GraphPad, San Diego).
Evaluation of Plasma Lipids.
Total cholesterol and triglycerides were determined by enzymatic methods in the Central Clinical Biochemistry Laboratory of the University Hospital Zurich with a Hitachi 747 analyzer.
Results
Impact of Hypercholesterolemia on Acute DC-Induced Arterial Inflammation.
In the transgenic SM-LacZ model of cardiovascular immunopathology, the β-gal antigen is immunologically ignored and β-gal-reactive T cells are not deleted because the antigen is expressed exclusively in nonlymphoid tissues. Immunization with β-gal-recombinant vaccinia virus or β-gal peptide-presenting DC (DC/β-gal) elicits artery- and heart-specific immune responses. Repetitive immunization of SM-LacZ mice with DC/β-gal leads to aggressive and destructive inflammation in the myocardium of the right heart and in small and large lung arteries (18). In addition, SM cells in the aortic sinus and more distal parts of the aorta are shown here to be targets for DC/β-gal-primed CD8 T cells (see below).
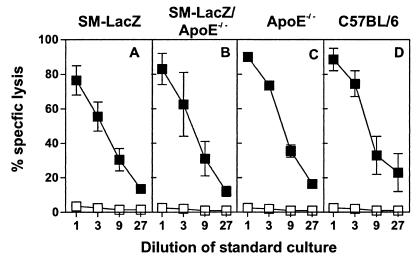
To investigate both the effect of hypercholesterolemia on the initiation of cytotoxic T lymphocyte-mediated arterial inflammation and the contribution of such localized arterial inflammatory reactions on the process of atherosclerosis, SM-LacZ mice were crossed with apoE−/− mice. Expression of the β-gal transgene in the vascular system of apoE−/− mice had no measurable influence on the hypercholesterolemic phenotype; plasma cholesterol and triglyceride concentrations were comparable in apoE−/− and SM-LacZ/apoE−/− mice (Table 1). T cell reactivity in the different mouse strains was not affected by the transgene expression in the vascular system or the hypercholesterolemia. This is shown by the fact that immunization with DC presenting the H2-Kb-binding β-gal-peptide elicited cytotoxic T lymphocyte responses in SM-LacZ (Fig. 1A), SM-LacZ/apoE−/− (Fig. 1B), or apoE−/− mice (Fig. 1C) that were similar to C57BL/6 control mice (Fig. 1D). Furthermore, in vivo T helper cell reactivity against β-gal also was unaffected in these mice as determined by immunization with β-gal protein and subsequent challenge with β-gal-coupled 2,4-dinitrophenyl; β-gal primed SM-LacZ, SM-LacZ/apoE−/−, apoE−/−, and C57BL/6 mice developed specific IgG anti-2,4-dinitrophenyl antibody responses that required primed β-gal-specific T help (not shown). Thus, the initial activation of artery-specific T cells was not influenced by high plasma cholesterol levels nor by the presence of the transgene in the vascular system.
Table 1.
Plasma cholesterol and triglyceride concentrations in SM-LacZ, apoE−/−, and SM-LacZ/apoE−/− mice
| Time postimmunization | Strain | Plasma
cholesterol and triglyceride, mmol/liter
|
|
|---|---|---|---|
| Cholesterol | Triglyceride | ||
| Day 0 | SM-LacZ | 2.1 ± 0.1 | 0.8 ± 0.1 |
| SM-LacZ/apoE−/− | 12.7 ± 1.1 | 1.1 ± 0.1 | |
| ApoE−/− | 10.9 ± 1.2 | 1.2 ± 0.1 | |
| Day 14 | SM-LacZ | 3.0 ± 0.2 | 0.8 ± 0.1 |
| SM-LacZ/apoE−/− | 11.5 ± 1.1 | 1.1 ± 0.1 | |
| ApoE−/− | 12.8 ± 1.3 | 1.2 ± 0.1 | |
| Day 35 | SM-LacZ | 3.3 ± 0.2 | 0.7 ± 0.1 |
| SM-LacZ/apoE−/− | 58.6 ± 2.4 | 1.0 ± 0.2 | |
| ApoE−/− | 56.7 ± 3.7 | 1.1 ± 0.3 | |
Mice were fed normal chow diet until day 14 and a diet enriched in fat and cholesterol from day 14 on. Mean values ± SEM of 6–10 mice per group are indicated.
Figure 1.
T cell immune responses against β-gal in hyper- and normocholesterolemic mice. Anti- β-gal cytotoxic T cell responses. SM-LacZ (A), SM-LacZ/apoE−/− (B), apoE−/− (C), or C57BL/6 (D) control mice were intravenously immunized with 3 × 105 DC/β-gal. Eight days later, spleen cells were restimulated in vitro for 5 d with peptide-labeled, irradiated spleen cells. Specific lysis was determined on β-gal497–504-pulsed EL4 target cells (■) or on EL4 cells without peptide (□). Mean ± SD of three mice per group are shown. Results from one of two comparable experiments are shown.
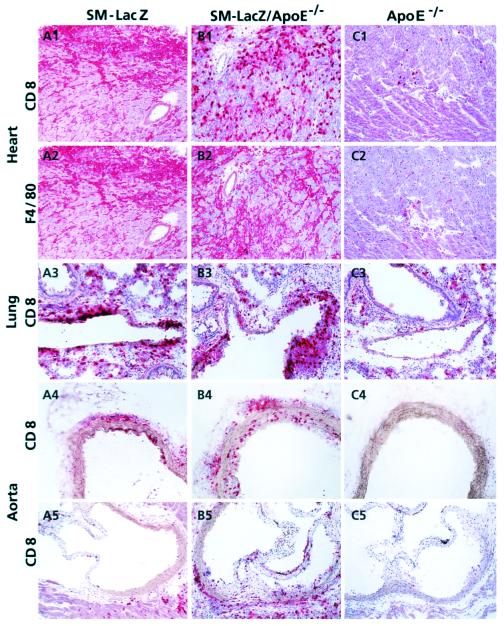
To induce strong sustained autoimmune responses against peripheral self-antigen that is normally ignored, repetitive injections of high doses of peptide presenting DC over a prolonged period are required (21). The protocol we used here consisted of six injections of 3 × 105 DC/β-gal on days 0, 1, 2, 8, 9, and 10 eliciting a destructive myocarditis with infiltration of CD8+ T cells and F4/80+ inflammatory macrophages on day 14 both in SM-LacZ (Fig. 2 A1 and A2) and SM-LacZ/apoE−/− mice (Fig. 2 B1 and B2). In contrast, hearts of apoE−/− mice did not show inflammatory infiltrates (Fig. 2 C1 and C2). Similarly, the quality of the infiltrate and the proportion of affected lung arteries in SM-LacZ (Fig. 2A3) and SM-LacZ/apoE−/− mice (Fig. 2B3) was comparable, whereas the lung arteries (Fig. 2C3) and the aorta (Fig. 2 C4 and C5) of apoE−/− and C57BL/6 control mice (not shown) remained free of inflammatory cells. The aorta of SM-LacZ mice, both in more distal locations in the aortic arch (Fig. 2A4) and the aortic sinus (Fig. 2A5), was less susceptible to the DC-induced immunopathology compared with SM-LacZ/apoE−/− mice (Fig. 2 B4 and B5). To quantitate the histological alterations present in heart, lung, and aorta, a scoring system ranging from 0 (no alteration) to 8 (complete loss of functional tissue) was established. The results of these analyses for the histological alterations are summarized in Table 2. Although the myocarditis and arteritis scores for lung arteries on day 14 were slightly higher in SM-LacZ/apoE−/− mice, the differences in comparison to SM-LacZ mice remained insignificant. A significant difference between the normocholesterolemic SM-LacZ and the hypercholesterolemic SM-LacZ/apoE−/− mice on day 14 was found in the inflammatory response in the aorta (Table 2). Taken together, these results show that hypercholesterolemia favored inflammatory responses in the vascular tree, particularly at ramifications, i.e., in locations where high shear-stress may act as an additional component for vascular injury.
Figure 2.
Immunohistological analysis of acute cardiovascular immunopathology. SM-LacZ (A1–A5), SM-LacZ/apoE−/− (B1–B5), or apoE−/− mice (C1–C5) were immunized repetitively with 3 × 105 β-gal 497–504-pulsed DC (days 0, 1, 2, 8, 9, and 10) and fed a normal chow diet. On day 14, frozen sections of heart tissue were stained for CD8 (A1, B1, and C1) and the macrophage marker F4/80 (A2, B2, and C2). Lung sections (A3, B3, and C3), sections from the aortic arch (A4, B4, and C4) and from the aortic sinus (A5, B5, and C5) were evaluated for the presence of acute lymphocyte infiltration by CD8 staining. (Original magnifications: ×100.)
Table 2.
Quantitative histological evaluation of immunopathological lesions in SM-LacZ, SM-LacZ/apoE−/−, and apoE−/− mice
| Strain/day | Histological
scores† in
|
Incidence of DCM‡ | ||
|---|---|---|---|---|
| Heart | Lung | Aorta | ||
| Day 14 postimmunization | ||||
| SM-LacZ | 5.3 ± 0.4 | 5.4 ± 0.5 | 1.8 ± 0.6 | 1/10 |
| SM-LacZ/apoE−/− | 6.5 ± 0.4 | 5.7 ± 0.3 | 4.7 ± 0.6* | 2/10 |
| ApoE−/− | 0.5 ± 0.3 | 0 | 0 | 0/4 |
| Day 35 postimmunization | ||||
| SM-LacZ | 4.2 ± 0.8 | 2.1 ± 0.5 | 1.3 ± 0.4 | 2/6 |
| SM-LacZ/apoE−/− | 4.3 ± 0.6 | 4.3 ± 0.6* | 3.1 ± 0.4* | 3/10 |
| ApoE−/− | 0 | 0 | 0 | 0/7 |
Mice were immunized with of 3 × 105 DC/β-gal on days 0, 1, 2, 8, 9, and 10 and fed normal chow diet until day 14 and a diet enriched in fat and cholesterol from day 14 on. Histological scores represent mean ± SEM of 6–10 mice per group determined as described in Materials and Methods. Significant differences (P < 0.05) between SM-LacZ and SM-LacZApoE−/− mice are indicated by an asterisk.
Dilated cardiomyopathy (DCM) was classified as substantial loss of functional heart tissue, thinning of the right ventricle wall, and dilatation of the right ventricle.
Arteritis Amplified Cholesterol-Induced Atherosclerosis.
We next assessed the chronic effect of linked immune-mediated arteritis and hypercholesterolemia and followed the development of atherosclerotic lesions in the aortic root. To this end, mice were immunized by using the same protocol of repetitive DC immunization and fed a diet enriched in fat and cholesterol from day 14 on. Both apoE−/− and SM-LacZ/apoE−/− mice developed elevated plasma cholesterol levels (Table 1), indicating that both the acute (day 14) and chronic (day 35) vascular immunopathology did not influence cholesterol metabolism.
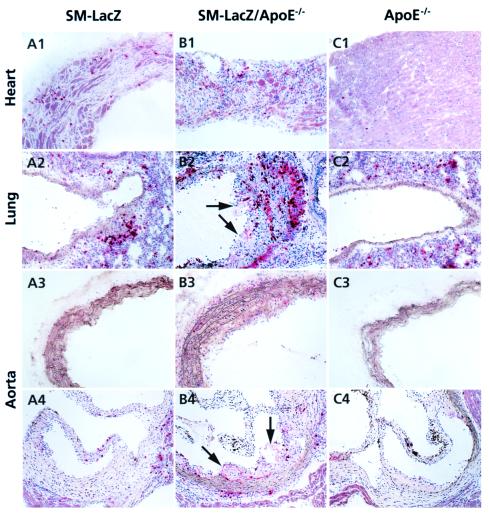
The destructive myocarditis in SM-LacZ mice can result in a dilated cardiomyopathy (18). In the present set of experiments, around 30% of both SM-LacZ and SM-LacZ/apoE−/− mice developed a pronounced dilatation of the right ventricle with loss of functional heart tissue (Table 2, Fig. 3 A1 and B1). In these and nondilated hearts (not shown), the acute CD8 T cell-dominated infiltration was replaced by a fibro-histiocytic infiltrate (Fig. 3 A1 and B1). The acute inflammatory lesions in lung arteries and the aorta of SM-LacZ mice nearly completely resolved with residual perivascular infiltrates (Fig. 3 A2–A4). In contrast, inflammatory reactions in lung arteries (Fig. 3B2) and aorta of SM-LacZ/apoE−/− mice resolved more slowly (Fig. 3 B2–B4) and were transformed into atherosclerotic lesions by the enforced hypercholesterolemia. The maintained inflammation often was associated with extensive intimal proliferation and foam cell accumulation (Fig. 3 B2 and B4, arrows). ApoE−/− mice, also in the long term, did not develop a vascular inflammatory disease in response to DC immunization (Fig. 3C, Table 2). The quantitative evaluation of the immunopathological lesions on day 35 showed that SM-LacZ, but not SM-LacZ/apoE−/−, mice recovered from the acute vascular inflammatory disease (Table 2). Taken together, these data show that hypercholesterolemia impairs the process of arterial recovery from immune-mediated injury and suggest that sites of arterial inflammation are more susceptible to cholesterol-induced lesion formation.
Figure 3.
Immunohistological evaluation of chronic inflammation and cholesterol-induced atherosclerosis. SM-LacZ (A1–A4), SM-LacZ/apoE−/− (B1–B4), or apoE−/− mice (C1–C4) were immunized as in Fig. 2 and kept for 14 days on normal chow diet followed by 3 weeks on a high-cholesterol diet. On day 35, frozen sections from heart (A1, B1, and C1), lung (A2, B2, and C2), aortic arch (A3, B3, and C3), and aortic sinus (A4, B4, and C4) were stained for CD8. Arrows in B2, B4, and C4 indicate development of atherosclerotic lesions in lung arteries and aortic sinus. (Original magnifications: ×100.)
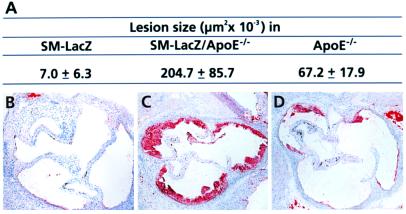
To assess the influence of arteritis on cholesterol-induced atherosclerosis in more detail, we quantitated the size of atherosclerotic lesions in the aortic root by using Oil Red O staining and computer-aided morphometry. Lipid deposition in the aortic root is a good measure for atherosclerosis because in apoE−/− mice the extent of atherosclerotic disease in the aortic root correlates well with that in the remainder of the aorta (22). As expected, SM-LacZ mice did not develop significant subintimal lipid accumulations (Fig. 4 A and B). Substantial lesion formation was found in both hypercholesterolemic SM-LacZ/apoE−/− (Fig. 4 A and C) and apoE−/− mice (Fig. 4 A and D), whereby SM-LacZ/apoE−/− mice showed a dramatically accelerated lesion formation in the chronically inflamed aorta (Fig. 4 A and C), indicating that the vascular inflammation favored the development of cholesterol-induced atherosclerosis.
Figure 4.
Evaluation of atherosclerotic lesions. DC/β-gal-immunized mice were fed normal chow diet for 14 days and high-cholesterol diet for additional 21 days. (A) The average size of atherosclerotic lesions on day 35. Statistical analysis using Student's t test revealed a highly significant difference (P < 0.001) for SM-LacZ/apoE−/− vs. apoE−/− mice. Representative sections stained with Oil Red O of (B) SM-LacZ, (C) SM-LacZ/apoE−/−, and (D) apoE−/− mice are shown. (Original magnifications in B–D: ×50.)
Discussion
This study documents the mutual effects of immune-mediated arteritis and hypercholesterolemia chronologically in a process leading to atherosclerotic arterial wall alterations. Using a transgenic mouse model of inducible cardiovascular immunopathology, we found that hypercholesterolemia selectively enhanced and perpetuated arterial, but not myocardial, inflammation. On the other hand, arterial inflammation significantly increased the susceptibility of the arterial wall to cholesterol-dependent atherosclerosis. Although immunopathology against ignored self-antigen seemed to dominate over effects of hypercholesterolemia early in the course of vascular disease (i.e., during days 0–14), it must be emphasized that the established lesions resolved within 2–3 wk in the absence of hypercholesterolemia. Importantly, the coexistence of hypercholesterolemia prevented recovery from arterial inflammation, supporting the notion that the mutual perpetuation of arteritis and cholesterol-induced atherosclerosis eventually might lead to a vicious circle of chronic vascular injury.
Promotion of Arterial Inflammation by Hypercholesterolemia.
Hypercholesterolemia and, in particular, the increase of modified LDL is a primary risk factor for atherosclerosis (2, 7). Modified LDL can be trapped by endothelial cells, vascular SM cells, and macrophages, which subsequently are activated to secrete proinflammatory cytokines and chemokines (5). Such initial triggering is thought to attract both T cells and macrophages to the site of arterial inflammation (8, 23). Intramural, activated T cells secreting cytokines (24) might further promote the inflammatory preatherosclerotic stage. However, studies in apoE−/− mice revealed that atherosclerotic lesions could also develop in the complete absence of lymphocytes (25) or under germ-free conditions (26), raising the question whether hypercholesterolemia-induced vascular immune reactions are merely nonspecific “bystander” phenomena. We believe that the results presented in this report help to resolve these discrepant observations. In accordance with previous reports (27), hypercholesterolemia in apoE−/− mice mediated only a mild vascular inflammation with very few infiltrating CD8 T cells. However, when hypercholesterolemia was linked with a specific immune response directed against a microbial antigen persisting in the vascular wall (SM-LacZ/apoE−/− mice), the early proinflammatory and disease-perpetuating effect of high plasma cholesterol levels became apparent. It is therefore most likely that hypercholesterolemia acts initially as a predisposing factor for specific acute inflammatory reactions in the vascular wall and subsequently helps to maintain these lesions in the chronic phase.
Mutual Chronic Perpetuation of Vascular Inflammation and Cholesterol-Induced Atherosclerosis—A Key Step in Atherogenesis?
Epidemiological data (9–11, 28) indicate the frequent infection of arteries with microbial pathogens. It is conceivable that successful immune responses against these pathogens are crucial to limit direct cytopathic and indirect immunopathological damage during vascular infections. Our transgenic model of inducible cardiovascular immunopathology facilitated investigation of such self-limiting antivascular inflammatory responses. Weak priming with DC (1–3 immunizations in the first week) induced acute myocarditis and arteritis in SM-LacZ mice without chronic sequelae in the myocardium or the arterial wall (B.L., unpublished results). Increasing dose and frequency of DC injections (≥ 6 injections in 2 weeks) exacerbated the disease, leading to a fulminant myocarditis with subsequent dilated cardiomyopathy (ref. 18 and this report) where cardiomyocytes were lost and replaced by nonfunctional fibro-histiocytic infiltrate. It is important to note that, in contrast to the progressive myocardial disease of SM-LacZ mice, the massive arteritis, both in lung arteries and the aorta, always resolved in the long term. This finding indicates the excellent regenerative capacity of the vascular wall of mice where proliferating SM cells and endothelial cells probably replace damaged cells and thereby maintain vessel integrity. However, these repair mechanisms apparently can be impaired by additional injuries, e.g., by hypercholesterolemia, which seems to amplify and maintain the antivascular immune response.
The response-to-injury hypothesis describes atherogenesis as a process of intimal thickening triggered by endothelial dysfunction (1). Beside the atherogenic effects of hypercholesterolemia, long-lasting exposure of the vascular wall to interferon-γ and probably other cytokines might induce SM cell proliferation and subsequent intimal thickening (29, 30). However, such strong effects of exclusively immune-mediated intima proliferation probably are restricted to posttransplant graft rejection and therefore are clearly distinct from the multigenic processes of atherosclerosis. Previous studies designed to dissect the complex pathogenesis of atherosclerosis have used C. pneumoniae infection of hypercholesterolemic apoE−/− (31,32) or LDLR−/− mice (33). These studies established that C. pneumoniae infects preferentially predamaged atheromatous areas of the aorta in severely hypercholesterolemic apoE−/− or LDLR−/− mice, but only very rarely healthy aortas of normocholesterolemic C57BL/6 mice (31) or aortas of hypercholesterolemic mice before development of cholesterol-induced lesions (33). It is therefore likely that a C. pneunomiae-induced local immune response in atherosclerotic arteries is an important aggravating factor in atherogenesis. This interpretation is supported by the presented data showing that local inflammatory reactions significantly accelerated hypercholesterolemia-induced atherosclerosis in classically predisposed (aorta) and other arterial sites.
Taken together, our study provides compelling evidence that the linkage of immune-mediated arterial inflammation and cholesterol-dependent effects critically contributes to the progressive nature of atherogenesis. Once a vicious circle of inflammation, deposition of lipids, and further inflammation is established, a self-perpetuating and progressive process of atherosclerotic lesion formation may develop. Thus, the experimental system used here appears to be a representative model for the complex mechanisms of atherogenesis and for evaluating strategies for preventing the advancement of atherosclerotic lesions.
Acknowledgments
We thank Drs. Kathy McCoy and Andrew Macpherson for helpful discussions and critical reading of the manuscript, Karin Brduscha-Riem for expert technical assistance, and Ida Schmieder and Norbert Wey for excellent photography. This work was supported by the Swiss National Science Foundation and the Kanton Zurich.
Abbreviations
- DC
dendritic cells
- apoE
apolipoprotein E
- apoE−/−
apoE-deficient
- LDL
low density lipoprotein
- SM
smooth muscle
- β-gal
β-galactosidase
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.220427097.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.220427097
References
- 1.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Braunwald E. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906. [DOI] [PubMed] [Google Scholar]
- 3.Ishibashi S, Goldstein J L, Brown M S, Herz J, Burns D K. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang S H, Reddick R L, Piedrahita J A, Maeda N. Science. 1992;258:468–471. doi: 10.1126/science.1411543. [DOI] [PubMed] [Google Scholar]
- 5.Terkeltaub R, Boisvert W A, Curtiss L K. Curr Opin Lipidol. 1998;9:397–405. doi: 10.1097/00041433-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Shattil S J, Ginsberg M H. J Clin Invest. 1997;100:S91–S95. [PubMed] [Google Scholar]
- 7.Steinberg D, Parthasarathy S, Carew T E, Khoo J C, Witztum J L. N Engl J Med. 1989;320:915–924. doi: 10.1056/NEJM198904063201407. [DOI] [PubMed] [Google Scholar]
- 8.Stemme S, Faber B, Holm J, Wiklund O, Witztum J L, Hansson G K. Proc Natl Acad Sci USA. 1995;92:3893–3897. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hendrix M G, Salimans M M, van Boven C P, Bruggeman C A. Am J Pathol. 1990;136:23–28. [PMC free article] [PubMed] [Google Scholar]
- 10.Chiu B, Viira E, Tucker W, Fong I W. Circulation. 1997;96:2144–2148. doi: 10.1161/01.cir.96.7.2144. [DOI] [PubMed] [Google Scholar]
- 11.Saikku P, Leinonen M, Mattila K, Ekman M R, Nieminen M S, Makela P H, Huttunen J K, Valtonen V. Lancet. 1988;2:983–986. doi: 10.1016/s0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- 12.Fabricant C G, Fabricant J, Litrenta M M, Minick C R. J Exp Med. 1978;148:335–340. doi: 10.1084/jem.148.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Q, Dietrich H, Steiner H J, Gown A M, Schoel B, Mikuz G, Kaufmann S H, Wick G. Arterioscler Thromb. 1992;12:789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- 14.George J, Shoenfeld Y, Afek A, Gilburd B, Keren P, Shaish A, Kopolovic J, Wick G, Harats D. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.atv.19.3.505. [DOI] [PubMed] [Google Scholar]
- 15.van Eden W. Immunol Rev. 1991;121:5–28. doi: 10.1111/j.1600-065x.1991.tb00821.x. [DOI] [PubMed] [Google Scholar]
- 16.Elias D, Markovits D, Reshef T, van der Zee R, Cohen I R. Proc Natl Acad Sci USA. 1990;87:1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moessler H, Mericskay M, Li Z, Nagl S, Paulin D, Small J V. Development (Cambridge, UK) 1996;122:2415–2425. doi: 10.1242/dev.122.8.2415. [DOI] [PubMed] [Google Scholar]
- 18.Ludewig B, Ochsenbein A F, Odermatt B, Paulin D, Hengartner H, Zinkernagel R M. J Exp Med. 2000;191:795–804. doi: 10.1084/jem.191.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ludewig B, Ehl S, Karrer U, Odermatt B, Hengartner H, Zinkernagel R M. J Virol. 1998;72:3812–3818. doi: 10.1128/jvi.72.5.3812-3818.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobbold S P, Jayasuriya A, Nash A, Prospero T D, Waldmann H. Nature (London) 1984;312:548–551. doi: 10.1038/312548a0. [DOI] [PubMed] [Google Scholar]
- 21.Ludewig B, Odermatt B, Ochsenbein A F, Zinkernagel R M, Hengartner H. Immunol Rev. 1999;169:45–54. doi: 10.1111/j.1600-065x.1999.tb01305.x. [DOI] [PubMed] [Google Scholar]
- 22.Tangirala R K, Rubin E M, Palinski W. J Lipid Res. 1995;36:2320–2328. [PubMed] [Google Scholar]
- 23.Xu Q B, Oberhuber G, Gruschwitz M, Wick G. Clin Immunol Immunopathol. 1990;56:344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- 24.Hansson G K, Holm J, Jonasson L. Am J Pathol. 1989;135:169– 175. [PMC free article] [PubMed] [Google Scholar]
- 25.Dansky H M, Charlton S A, Harper M M, Smith J D. Proc Natl Acad Sci USA. 1997;94:4642–4646. doi: 10.1073/pnas.94.9.4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright S D, Burton C, Hernandez M, Hassing H, Montenegro J, Mundt S, Patel S, Card D J, Hermanowski-Vosatka A, Bergstrom J D, et al. J Exp Med. 2000;191:1437–1442. doi: 10.1084/jem.191.8.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roselaar S E, Kakkanathu P X, Daugherty A. Arterioscler Thromb Vasc Biol. 1996;16:1013–1018. doi: 10.1161/01.atv.16.8.1013. [DOI] [PubMed] [Google Scholar]
- 28.Adam E, Melnick J L, Probtsfield J L, Petrie B L, Burek J, Bailey K R, McCollum C H, DeBakey M E. Lancet. 1987;2:291–293. doi: 10.1016/s0140-6736(87)90888-9. [DOI] [PubMed] [Google Scholar]
- 29.Russell P S, Chase C M, Winn H J, Colvin R B. Transplantation. 1994;57:1367–1371. [PubMed] [Google Scholar]
- 30.Tellides G, Tereb D A, Kirkiles-Smith N C, Kim R W, Wilson J H, Schechner J S, Lorber M I, Pober J S. Nature (London) 2000;403:207–211. doi: 10.1038/35003221. [DOI] [PubMed] [Google Scholar]
- 31.Moazed T C, Kuo C, Grayston J T, Campbell L A. J Infect Dis. 1997;175:883–890. doi: 10.1086/513986. [DOI] [PubMed] [Google Scholar]
- 32.Moazed T C, Campbell L A, Rosenfeld M E, Grayston J T, Kuo C C. J Infect Dis. 1999;180:238–241. doi: 10.1086/314855. [DOI] [PubMed] [Google Scholar]
- 33.Hu H, Pierce G N, Zhong G. J Clin Invest. 1999;103:747–753. doi: 10.1172/JCI4582. [DOI] [PMC free article] [PubMed] [Google Scholar]