Abstract
Purpose
Although previous studies have indicated that elevated levels of the tissue plasminogen activator (tPA) and the urokinase plasminogen activator (uPA) associate with the death of retinal ganglion cells (RGCs), it was unclear whether these proteases directly cause cell death. With the use of a transformed and undifferentiated retinal ganglion cell line, RGC-5, which does not express tPA, and by treating this cell line with staurosporine, which induces not only the differentiation of RGC-5 cells but also the expression of uPA and tPA in other neuronal cells, the authors sought to determine whether these proteases regulate the differentiation of RGC-5 cells and whether elevated levels of these proteases directly cause the death of RGC-5 cells.
Methods
Transformed RGC-5 cells were cultured in serum-free medium and were treated with 0.5 μM to 2.0 μM staurosporine to induce their differentiation. Neurite outgrowth was assessed by phase-contrast microscopy and calcein AM staining and quantified with imaging software. Proteolytic activities of tPA and uPA were determined by zymography assays. Cell viability was determined by LIVE/DEAD viability assay kit.
Results
Compared with untreated RGC-5 cells, cells treated with staurosporine differentiated as early as 1 to 6 hours. However, proteolytic activities of neither tPA nor uPA were observed within this time frame. Differentiated RGC-5 cells expressed detectable levels of uPA proteolytic activity starting at 24 hours and tPA proteolytic activity only at 48 hours. RGC-5 cells synthesized and secreted uPA and tPA into the conditioned medium, depending on staurosporine concentration and treatment time. At lower concentrations of staurosporine, differentiated RGC-5 cells had longer neurites and expressed lower levels of tPA and uPA. At higher concentrations of staurosporine, differentiated RGC-5 cells expressed higher levels of tPA and uPA, had smaller neurites, and most of them died. In contrast, when RGC-5 cells were treated with staurosporine along with inhibitors specific to tPA and uPA, proteolytic activities of both PAs were significantly reduced. Under these conditions, a significant number of RGC-5 cells survived, showed increased neurite outgrowth, and established their neurite network in vitro.
Conclusions
Results presented in this study indicate that RGC-5 cells do not require tPA and tPA for their differentiation.
Ganglion cells are the only cells from the retina that physically interact with the central nervous system, and they play a critical role in transmitting light signals to visual processing centers in the brain. In a number of neurodegenerative conditions including glaucoma,1–3 these terminally differentiated retinal ganglion cells (RGCs) undergo cell death.4–7 However, the mechanisms underlying the death of RGCs are still poorly understood.
We have previously reported that under certain glaucoma-related degenerative conditions, increased levels of two plasminogen activators (PAs), the tissue plasminogen activator (tPA) and the urokinase type plasminogen activator (uPA), promoted the death of RGCs. First, we reported that retinal ischemia, induced by the ligation of the optic nerve in mice, leads to elevated levels of tPA and uPA in the ganglion cell layer of the retina, and that elevated levels of these proteases, in turn, promote the death of RGCs.8 Second, we reported that hyperstimulation of retinal glutamate receptors in mice (excitotoxicity) also leads to elevated levels of tPA and uPA and that these proteases, in turn, promote the death of RGCs.9 In addition, recent studies from other laboratories have reported that the same proteases promote the death of RGCs in response to hyperstimulation of glutamate receptors.10,11 Despite this evidence, it is still unclear whether these proteases directly cause the death of RGCs. Therefore, we explored an in vitro system in which RGCs are devoid of tPA and uPA and in which levels of both these proteases can be controlled.
Despite the fact that primary cultures of RGCs are appropriate for in vitro studies, primary RGCs survive for few generations in vitro and their isolation from retinas is cumbersome. In addition, normal adult RGCs express low levels of tPA proteolytic activity constitutively,9 whereas our study required ganglion cells devoid of PAs. Previous studies have used PC12 cells, in which tPA levels can be induced, but these cells are originally derived from a rat pheochromocytoma,12 not from the normal retina, and these cells do not share characteristic features of RGCs. Recent studies indicated that a rat transformed ganglion cell line, RGC-5, has a number of characteristic features of normal RGCs, including the expression of Thy-1, Brn-3c, and glutamate receptors.13 Although RGC-5 cells can be used as a substitute for primary RGCs, these cells proliferate in vitro and exhibit morphology similar to that of fibroblasts. RGC-5 cells, nevertheless, can be differentiated into neuronlike (and nonmitotic) cells by treatment with a nonspecific kinase inhibitor, staurosporine.14 Furthermore, we observed that undifferentiated RGC-5 cells do not express tPA proteolytic activity constitutively; therefore, these cells seemed suitable for our study. Because previous studies on PC12 cells indicated that staurosporine induces tPA proteolytic activity15 and because undifferentiated RGC-5 cells do not express tPA proteolytic activity, we used RGC-5 cells to modulate proteolytic activities of tPA and uPA and determined whether these proteases play a direct role in differentiation and in cell death. Here, we report that elevated levels of tPA and uPA, indeed, play a direct role in the death of RGC-5 cells.
Materials and Methods
Materials
The following materials were obtained for use in this study: Dulbecco modified Eagle medium (DMEM), Dulbecco phosphate-buffered saline (DPBS), penicillin, and streptomycin (Invitrogen, Carlsbad, CA); staurosporine (Alexis Biochemicals, San Diego, CA); human glu-plasminogen (product 410), human fibrinogen (product 431), recombinant plasminogen activator inhibitor (rPAI-1; product 104) and tPA-STOP (2,7-bis-[4-amidinobenzylidene]-cycloheptanone-1 dihydrochloride; product 544), 2-chain recombinant tPA (product 174), and human urokinase (product 124; American Diagnostica, Stamford, CT); amiloride (Sigma Chemical Co., St. Louis, MO); and the LIVE/DEAD viability kit (Molecular Probes, Eugene, OR).
Cell Culture
RGC-5 cells were cultured in DMEM containing 1 g/L glucose and supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37°C in a humidified incubator, circulated with 5% CO2. RGC-5 cells were routinely cultured in 100-mm tissue culture plates with DMEM containing 10% FBS. For the indicated experiments, RGC-5 cells were trypsinized, and cell number was determined with the use of a Coulter counter and plated at a density of 4 × 103 cells/mL in 96-well tissue culture plates; 100 μL cell suspension was placed in each well. RGC-5 cells were cultured in this fashion overnight. The next morning, medium was aspirated, cells were washed twice with DPBS to remove serum, and cells were incubated with staurosporine in DMEM containing no FBS. Where indicated, RGC-5 cells were also treated with indicated concentrations of tPA-STOP (which inhibits tPA proteolytic activity) or amiloride (which inhibits uPA proteolytic activity) in DMEM containing no FBS.
Cell Morphology
Cell morphology was assessed with the use of an inverted, phase-contrast, bright-field microscope. Digitized images were obtained with a digital camera (Nikon, Tokyo, Japan) and saved as jpg files. Neurite outgrowth of RGC-5 cells was assessed (NeuronJ image software, version 1.36b; http://rsb.info.nih.gov/ij). For purposes of measurement, we defined neurites as projections from the cells that were as long as or longer than one cell diameter. Neurite outgrowth was measured only from staurosporine-treated cells, not from cells left untreated. Forty cells from each condition were analyzed according to previously published methods.14 The average length of neurites from three independent experiments was expressed as the mean ± SEM.
Cell Death
Cell viability was determined with the LIVE/DEAD viability assay kit. This assay kit determines live and dead cells, simultaneously, based on intracellular esterase activity of live cells and plasma membrane integrity of dead cells. Briefly, cells plated in eight-well chamber slides or on sterile glass coverslips (2 × 103 cells/mL) were incubated (without fixation) at room temperature for 30 minutes with a mixture of solution containing 2 μM calcein AM and 4 μM ethidium bromide prepared in tissue culture-grade D-PBS. At the end of the incubation period, the gasket from and eight-well chamber slide was removed, a drop of aqueous mounting was added, and a coverslip was placed on the slides. When cells were plated on coverslips, the coverslips were inverted with a fine-tipped forceps and were mounted on microscope slides with a drop of aqueous mounting medium. Slides were then observed under a bright-field microscope (Nikon) equipped with epifluorescence. Live and dead cells were individually counted from a total of at least 80 to 100 cells (from each condition) and were represented as mean ± SEM. Statistical significance was analyzed with a nonparametric Newman-Keuls analog procedure (GB-Stat Software; Dynamic Microsystems, Silver Spring, MD). Micrographs shown in Figure 6B were obtained with a SPOT digital camera. Color images were converted to grayscale with commercial software (Adobe Photoshop, versions 5.5 and 7.0; Adobe System Inc., Mountain View, CA).
Figure 6.
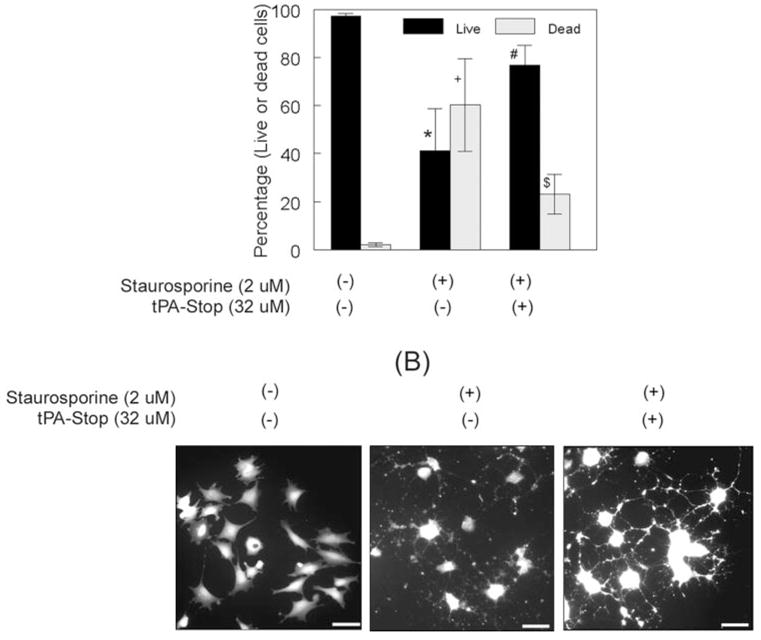
Inhibition of tPA and uPA proteolytic activity attenuated the death of RGC-5 cells and stabilized their neurite network. RGC-5 cells (2 × 103 cells/mL) were left untreated or were treated for 48 hours with 2.0 μM staurosporine and 32 μM tPA-STOP. (A) Quantification of live and dead cells indicated that most RGC-5 cells left untreated survived but that a significant number of cells died after staurosporine treatment. A significant number of RGC-5 cells survived when treated with staurosporine along with tPA-STOP. *+P < 0.05, compared with untreated cells. #$P < 0.05, compared with staurosporine-treated cells. (B) Calcein AM staining indicated that RGC-5 cells left untreated were fibroblastic, without neurite outgrowth. RGC-5 cells treated with staurosporine alone differentiated, but their neurites appeared fragmented. In contrast, RGC-5 cells treated with 32 μM tPA-STOP and 2.0 μM staurosporine differentiated, but their neurites appeared smooth, and they formed a network with neighboring cells. Scale bar, 40 μm.
Zymography
Proteolytic activity of tPA and uPA was determined by substrate zymography according to methods previously described.8,9 Briefly, aliquots containing equal amounts of conditioned medium (20 μL) or cell extracts (25 μg total protein; in a buffer containing 1% nonidet-P40, 20 mM Tris-HCl, 150 mM NaCl [pH 7.4]), collected at defined intervals after each treatment, were mixed with 4× SDS gel-loading buffer and were loaded without reduction or heating onto 10% SDS polyacrylamide gels containing fibrinogen (5.5 mg/mL) and plasminogen (50 μg/mL). After electrophoresis, the gels were washed three times with 2.5% Triton X-100 (15 minutes each time), placed in 0.1 M glycine-buffer (pH 8.0), and incubated overnight at 37°C to allow proteolysis of the substrates in the gels. After overnight incubation, the gels were stained with 0.2% Coomassie brilliant blue R250 and then were destained with a solution containing 50% methanol and 10% acetic acid in deionized water. Samples containing standard recombinant tPA or uPA were electrophoresed for comparison (data not shown). tPA activity in zymograms was confirmed by incubation of the gels with the tPA inhibitor tPA-STOP (data not shown). uPA activity was confirmed by incubation of the gels with the uPA inhibitor amiloride (data not shown).
Results
Effect of Staurosporine on Neurite Outgrowth in RGC-5 Cells
Previous studies by Frasetto et al.14 have reported that staurosporine, in a dose-dependent fashion, induces the differentiation and neurite outgrowth of RGC-5 cells. Based on their studies, we treated RGC-5 cells with 0.5 μM, 1.0 μM, and 2.0 μM staurosporine in serum-free medium (because serum contains low levels of tPA and uPA) for 1 hour. Consistent with previous studies, staurosporine modulated the morphology of RGC-5 cells (Fig. 1A) and induced their neurite outgrowth in a dose-dependent fashion (Fig. 1B).
Figure 1.
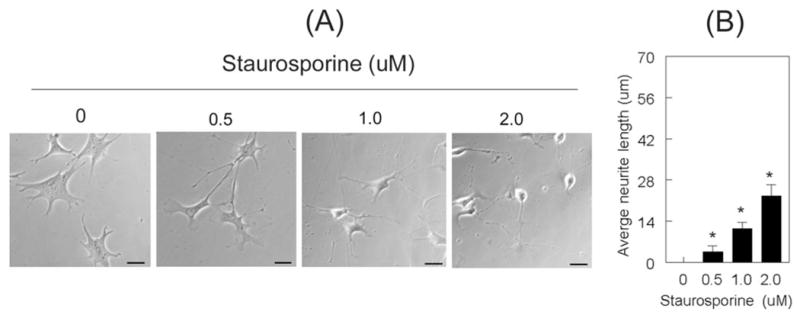
Staurosporine induced the differentiation of RGC-5 cells. RGC-5 cells (2 × 103 cells/mL) were left untreated or were treated with indicated concentrations of staurosporine (in serum-free medium), and their morphology was observed 1 hour after treatment. Neurite length at each concentration was measured, and length from 40 cells (from three independent experiments) was expressed as mean ± SEM (A) Increasing concentrations of staurosporine induced the differentiation of RGC-5 cells and (B) led to a dose-dependent increase in neurite outgrowth. *P < 0.05, compared with undifferentiated cells. Scale bar, 40 μm.
Effect of Staurosporine on uPA and tPA Proteolytic Activity
Because we reasoned that RGC-5 cells require tPA and uPA for differentiation and neurite outgrowth, we treated RGC-5 cells with 2.0 μM staurosporine (in serum-free medium) and incubated them at 37°C for 6 hours, 24 hours, and 48 hours. At the end of each incubation period, we collected the conditioned medium and determined proteolytic activities of tPA and uPA by zymography assay. We chose 2.0 μM staurosporine because this concentration induced significant neurite outgrowth as early as 1 hour (Fig. 1). Although staurosporine induced the neurite outgrowth of RGC-5 cells by 6 hours (Figs. 2A, 2B), zymography assays did not detect proteolytic activities of tPA or uPA in the conditioned medium (secreted) at this time point (Fig. 2C upper panel); they did synthesize low levels of uPA (cell-bound; Fig. 2C lower panel). At 24 hours, staurosporine caused a reduction of neurite outgrowth in RGC-5 cells, and induced the levels of cell-bound and secreted uPA, but not of tPA (Fig. 2C). At 48 hours, staurosporine caused a further reduction of neurite outgrowth in RGC-5 cells (Figs. 2A, 2B), and an induction in the levels of cell-bound (Fig. 2C, lower panel) and secreted uPA (Fig. 2C, upper panel). In addition, staurosporine induced the synthesis and secretion of tPA (Figs. 2C, 2D) at 48 hours. RGC-5 cells left untreated did not differentiate or show detectable levels of cell-bound and secreted tPA at each time point tested (Fig. 2F). Undifferentiated RGC-5 cells, nevertheless, synthesized low levels of uPA constitutively and secreted it into the medium starting at 24 hours. RGC-5 cells, when treated with dimethyl sulfoxide (DMSO) used to dissolve staurosporine, neither differentiated nor expressed tPA (data not shown). These results indicate that proteolytic activities of tPA and uPA were not required for the differentiation of RGC-5 cells because these cells had already differentiated by 6 hours, but did not synthesize tPA or uPA at this time point.
Figure 2.
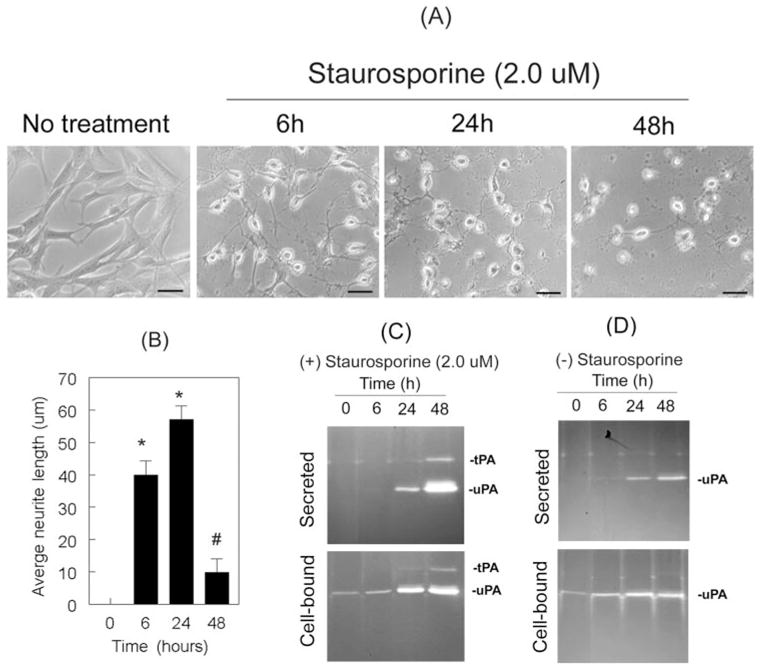
Differentiation of RGC-5 cells was associated with the expression of tPA and uPA. RGC-5 cells (4 × 103 cells/mL) were left untreated or were treated with 2.0 μM staurosporine (in serum-free medium). At 6 hours, 24 hours, and 48 hours after treatment, their morphology was observed under a phase-contrast microscope. Conditioned medium (CM) was collected from untreated and treated cells, and proteolytic activities of tPA and uPA were determined by zymography assays. (A) Staurosporine induced the differentiation of RGC-5 cells in a time-related fashion and (B) led to an increase in neurite length at 6 hours and 24 hours (scale bar, 40 μm). At 48 hours, however, RGC-5 cells had reduced neurite length. *P < 0.05, compared with untreated cells. #P < 0.05, compared with cells treated for 24 hours. (C) At 6 hours, zymography assays did not detect proteolytic activity of uPA or tPA, though RGC-5 cells had already differentiated. Differentiated RGC-5 cells synthesized uPA (C, lower panel) and secreted it into the conditioned medium at 24 hours (C, upper panel). At 48 hours they also expressed tPA. Undifferentiated RGC-5 cells did not express tPA, but they expressed uPA.
Given that the above experiments were performed with 2.0 μM staurosporine, we performed additional experiments to determine whether staurosporine induces the proteolytic activities of tPA and uPA based on its concentration. RGC-5 cells were treated for 48 hours with 0.5 μM, 1.0 μM, and 2.0 μM staurosporine, and the differentiation of RGC-5 cells was observed by light microscopy. In addition, conditioned medium was collected to determine the proteolytic activities of uPA and tPA. Differentiation and neurite outgrowth were observed in RGC-5 cells treated with 0.5 μM staurosporine but not in cells left untreated (Figs. 3A, 3B). At this concentration, RGC-5 cells expressed relatively higher levels of uPA than was observed in untreated cells (Fig. 3C), and they also expressed low levels of tPA. RGC-5 cells treated with 1.0 μM staurosporine showed reduced neurite outgrowth compared to 0.5 μM staurosporine-treated cells. Reduced neurite outgrowth at 1.0 μM concentration was associated with higher levels of tPA and uPA compared with RGC-5 cells treated with 0.5 μM staurosporine (Fig. 3C). RGC-5 cells treated with 2.0 μM staurosporine showed a further reduction in neurite outgrowth, and they also expressed additional increase in proteolytic activities of tPA and uPA.
Figure 3.
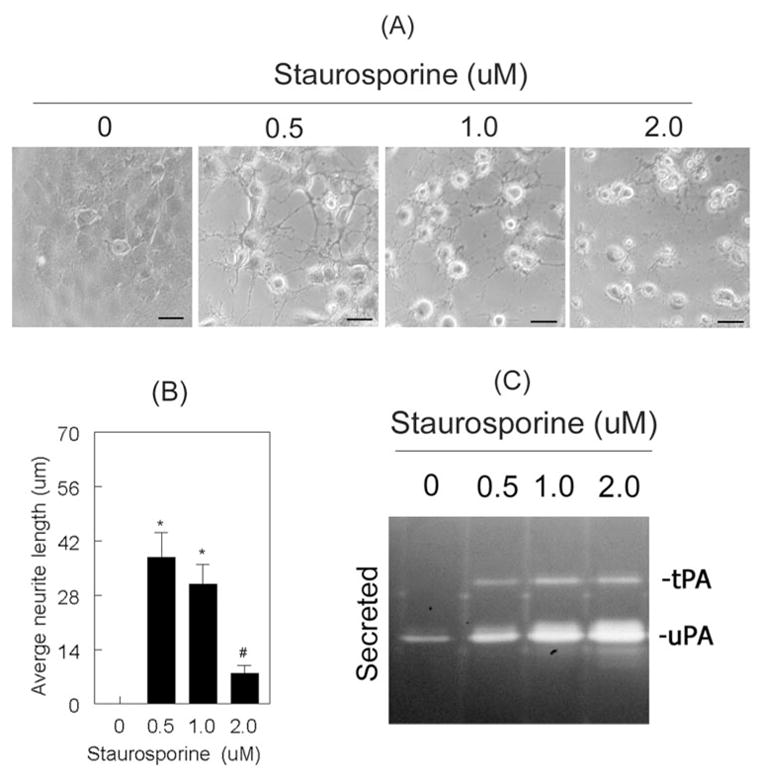
Staurosporine induced proteolytic activities of tPA and uPA in a dose-dependent fashion. RGC-5 cells (4 × 103 cells/mL) were left untreated or were treated for 48 hours with 0.5 μM, 1.0 μM, and 2.0 μM staurosporine (in serum-free medium). (A) RGC-5 cells treated with staurosporine (up to 1.0 μM) differentiated and had increased neurite outgrowth. However, at a concentration of 2.0 μM, neurite outgrowth was significantly reduced (B). Scale bar, 40 μm. *P < 0.05, compared with untreated cells. #P < 0.05, compared with 0.5 μM staurosporine-treated cells. (C) RGC-5 cells left untreated expressed low levels of uPA but not tPA, whereas cells treated with staurosporine expressed increased levels of both tPA and uPA.
Effect of uPA Inhibition on Neurite Outgrowth
Because our results thus far indicated that increased proteolytic activities of uPA and tPA reduced neurite outgrowth, we sought to determine whether inhibiting these proteases would reverse the process. First, we determined—with the use of amiloride, an inhibitor that specifically inhibits the proteolytic activity of uPA—the effect of uPA inhibition on RGC-5 cells. RGC-5 cells were left untreated or were treated for 48 hours with 2.0 μM staurosporine, 2.0 μM staurosporine and 200 μM amiloride, or 2.0 μM staurosporine and 400 μM amiloride. Morphologic examination indicated that RGC-5 cells treated with 2.0 μM staurosporine alone differentiated but that neurite outgrowth was reduced (Figs. 4A, 4B). RGC-5 cells treated with 400 μM amiloride and 2.0 μM staurosporine, however, showed an increase in neurite outgrowth, whereas RGC-5 cells treated with 200 μM amiloride and 2.0 μM staurosporine showed relatively smaller neurites. To determine whether the increase in neurite length observed in RGC-5 cells treated with amiloride resulted from reduced uPA proteolytic activity, conditioned medium collected at 48 hours after incubation was subjected to zymographic examination (Fig. 4C). Conditioned medium collected from RGC-5 cells left untreated showed low levels of uPA alone, whereas conditioned medium from RGC-5 cells treated with 2.0 μM staurosporine showed increased levels of uPA and tPA, as expected (Fig. 4C). Conditioned medium collected from RGC-5 cells treated with 200 μM and 400 μM amiloride along with 2.0 μM staurosporine showed a progressive reduction in proteolytic levels of uPA. Conditioned medium collected from undifferentiated RGC-5 cells treated with amiloride, without staurosporine, also showed a decrease in uPA proteolytic activity (constitutively observed), but this decrease had no effect on RGC-5 cell morphology (data not shown). Although amiloride concentrations greater than 400 μM might decrease the proteolytic activity of uPA further, we could not test additional concentrations because of solubility issues. Overall, these results indicated that amiloride inhibited staurosporine-induced proteolytic activity of uPA and increased neurite outgrowth in RGC-5 cells.
Figure 4.
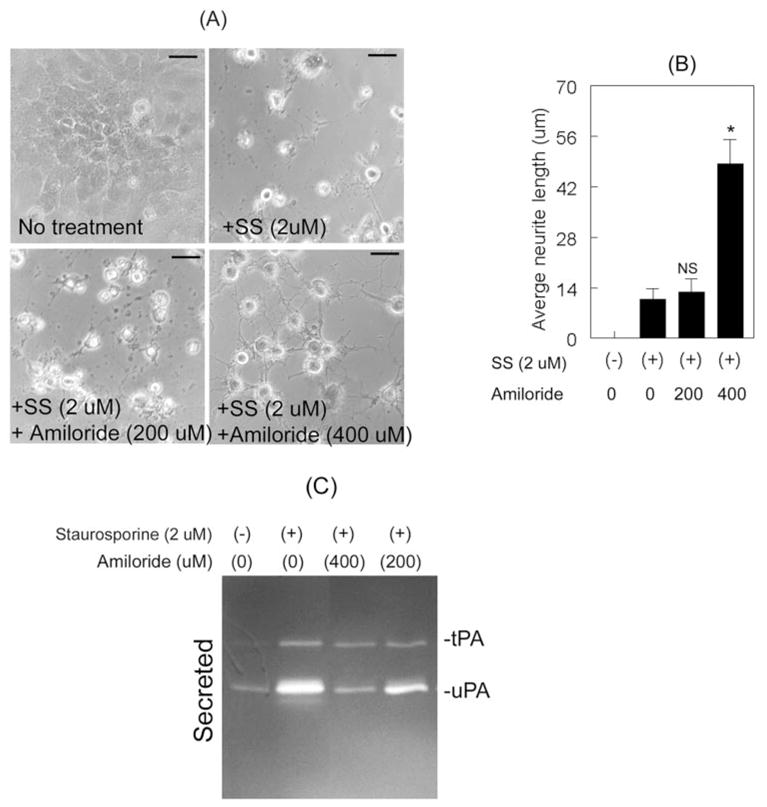
Inhibition of uPA proteolytic activity increased neurite outgrowth. RGC-5 cells (4 × 103 cells/mL) were left untreated or were treated for 48 hours with 2.0 μM staurosporine (SS) or with 2.0 μM staurosporine (SS) and indicated concentrations of amiloride. (A, B) Compared with untreated cells, staurosporine-treated cells differentiated but neurites were shorter. RGC-5 cells treated with 2.0 μM staurosporine and 400 μM amiloride also differentiated, but these cells exhibited increased neurite length than did cells treated with 2.0 μM staurosporine alone or cells treated with staurosporine and 200 μM amiloride. Scale bar, 40 μm. *P × 0.05, compared with cells treated with staurosporine alone (NS). (C) CM collected from untreated cells indicated low levels of uPA, whereas CM collected from staurosporine-treated cells showed increased levels of tPA and uPA. In contrast, CM collected from cells treated with 400 μM amiloride along with staurosporine showed a reduction in the proteolytic activity of uPA.
Effect of tPA Inhibition on Neurite Outgrowth
To determine whether the inhibition of tPA proteolytic activity also leads to an increase neurite outgrowth, we used tPA-STOP, which inhibits the proteolytic activity of tPA. RGC-5 cells were left untreated or were treated for 48 hours with 2.0 μM staurosporine or with 4 μM, 8 μM, 16 μM, and 32 μM tPA-STOP and 2.0 μM staurosporine. Light microscopy observations made at 48 hours after treatment indicated that RGC-5 cells treated with staurosporine differentiated but that they also had reduced neurite outgrowth (Figs. 5A, 5B). In contrast, RGC-5 cells treated with increasing concentrations of tPA-STOP showed a progressive increase in neurite outgrowth (Figs. 5A, 5B). To determine whether increased neurite outgrowth could be attributed to an inhibition in tPA proteolytic activity, conditioned medium collected at 48 hours after treatment was subjected to zymography. Conditioned medium collected from staurosporine-treated RGC-5 cells showed increased levels of uPA and tPA compared with the cells left untreated (Fig. 5C). However, conditioned medium collected from cells treated with increasing concentrations of tPA-STOP and 2.0 μM staurosporine showed dose-dependent reductions in proteolytic activity of tPA (Fig. 5C). In fact, conditioned medium collected from RGC-5 cells treated with 2.0 μM staurosporine and 32 μM tPA-STOP did not show proteolytic activity of tPA or uPA. This finding was expected because, at this high dose, tPA-STOP inhibits both tPA (Ki, 0.035 μM for tPA), and uPA (Ki, 3.4 μM for uPA). Conditioned medium from undifferentiated RGC-5 cells treated with tPA-STOP (without staurosporine) did not show tPA proteolytic activity, and tPA-STOP-treatment had no effect on RGC-5 cell morphology (data not shown). Although treatment of RGC-5 cells with amiloride and tPA-STOP might have had an additive effect, we did not perform additional experiments to determine this possibility because at 32 μM tPA-STOP completely inhibited the proteolytic activities of tPA and uPA.
Figure 5.
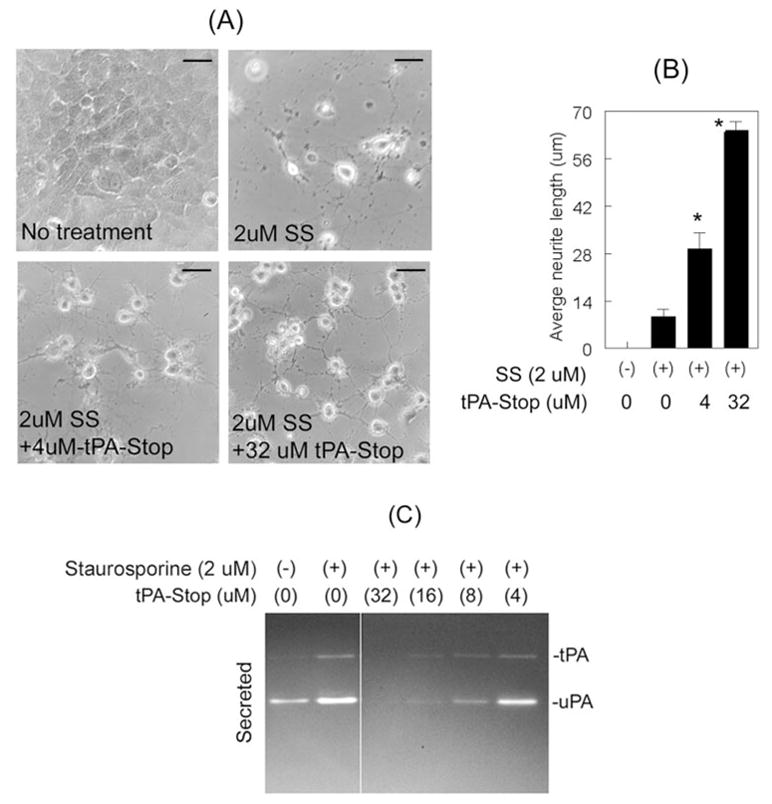
Inhibition of tPA proteolytic activity increased neurite outgrowth. RGC-5 cells (4 × 103 cells/mL) were left untreated or were treated for 48 hours with 2.0 μM staurosporine (SS) or with 2.0 μM staurosporine (SS) and indicated concentrations of tPA-STOP. (A, B) Compared with cells left untreated, RGC-5 cells treated with staurosporine differentiated but they also had shorter neurites. RGC-5 cells treated with 32 μM tPA-STOP and staurosporine differentiated and had longer neurites than cells treated with staurosporine alone. Scale bar, 40 μm. *P < 0.05, compared with cells treated with staurosporine alone. (C) CM collected from untreated cells indicated low levels of uPA, whereas CM collected from staurosporine-treated cells showed increased levels of tPA and uPA. In contrast, CM collected from cells treated with staurosporine and 4 μM to 32 μM tPA-STOP showed reduced proteolytic activities of both tPA and uPA in a dose-dependent fashion.
Effect of uPA and tPA Inhibition on Cell Death and Neurite Network
In the present study, RGC-5 cells treated with 2.0 μM staurosporine expressed elevated levels of tPA and uPA proteolytic activity, showed reduced neurite outgrowth, and seemed to be undergoing cell death (Figs. 2A, 3A, 4A, 5A). Therefore, we performed additional experiments to determine whether RGC-5 cells underwent cell death because of increased levels of proteases and whether the inhibition of proteolytic activities of uPA and tPA attenuates cell death. RGC-5 cells were left untreated or were treated for 48 hours with 2.0 μM staurosporine or 2.0 μM staurosporine along with 32 μM tPA-STOP. We used 32 μM tPA-STOP because this concentration inhibited the proteolytic activities of both tPA and uPA (Fig. 5C). At the end of 48 hours, RGC-5 cells were incubated for 30 minutes with a mixture containing calcein AM (which detects live cells) and ethidium bromide (which detects dead cells) and were examined under a fluorescence microscope (calcein AM and ethidium bromide were components of the LIVE/DEAD cytotoxicity kit). The number of cells showing calcein AM fluorescence and the number of cells showing ethidium bromide were then counted, and the results were expressed as mean number of cells ± SEM (Fig. 6A). Most RGC-5 cells left untreated showed calcein AM fluorescence (an indication that these cells survived), but few cells showed ethidium bromide fluorescence (an indication of dead cells). Further analysis indicated that approximately 40% of RGC-5 cells treated with staurosporine alone underwent cell death. In contrast, a significant number of RGC-5 cells survived after treatment with 32 μM tPA-STOP and 2.0 μM staurosporine (Fig. 6A). RGC-5 cell death did not result simply from the absence of serum, as previously described,16 because no significant cell death was observed when RGC-5 cells were left in serum-free medium for 48 hours (Fig. 6A).
To determine whether RGC-5 cells treated with 32 μM tPA-STOP and 2.0 μM staurosporine also establishes a network with neighboring cells, RGC-5 cells were treated for 48 hours, as described, and at the end of the treatment period they were incubated (without any fixation) with calcein AM (2 μM final concentration) for 1 hour at room temperature and were observed under a fluorescence microscope. RGC-5 cells left untreated showed calcein AM fluorescence in cell soma, but they were undifferentiated (Fig. 6B). RGC-5 cells treated with staurosporine alone showed calcein AM fluorescence in cell soma and in neurites. However, their neurites were fragmented, and the cells failed to make proper synapses with neighboring cells. In contrast, RGC-5 cells treated with staurosporine along with 32 μM tPA-STOP had intact neurites, and they also formed a synaptic network with neighboring cells. Results of this study indicate that the inhibition of proteolytic activities of tPA and uPA promotes the survival of RGC-5 cells and stabilizes their neurite network.
Discussion
In this study, we used transformed RGC-5 cells, which do not express tPA proteolytic activity constitutively and allowed them to secrete increased levels of tPA and uPA by treatment with specific doses of staurosporine. We then showed, for the first time, that RGC-5 cells secrete elevated levels of tPA and uPA and that these proteases play a direct role in RGC-5 cell death. We found that expression of these proteases is not required for the differentiation of RGC-5 cells; rather, cells expressed increased levels tPA and uPA proteolytic activity as a consequence of their differentiated phenotype. At a moderate concentration of staurosporine (0.5 μM), RGC-5 cells differentiated at a slower rate and secreted lower levels of tPA and uPA. At a higher concentration of staurosporine (2.0 μM), RGC-5 cells differentiated rapidly, but they also secreted higher levels of tPA and uPA. In the presence of these higher tPA and uPA levels, a significant number of RGC-5 cells also died (we have not determined whether cells underwent apoptotic and necrotic cell death). In contrast, inhibition of tPA (by tPA-STOP) and uPA (by amiloride) led to a significant attenuation in RGC-5 cell death. Under these conditions, RGC-5 cells also established a neurite network with neighboring cells.
An accepted model for the role of PAs is that PAs convert an inactive plasminogen (mostly present in capillaries) to active plasmin, and plasmin, in turn, degrades the extracellular matrix (ECM) to facilitate the elongation of newly formed neurites under physiological conditions.17–22 Uncontrolled plasminogen activation, however, also leads to cell death under pathologic conditions in the central nervous system23–25 and in the retina.8,10,11 Furthermore, tPA and uPA can promote the death of RGCs by two different mechanisms, one plasminogen dependent8 and the other plasminogen independent.9–11 In the present study, elevated levels of tPA and uPA seemed to promote the death of RGC-5 cells independently of plasminogen activation because we did not observe plasminogen activation by casein zymography assays (data not shown). We also observed some differences between transformed RGC-5 cells and normal RGCs regarding the expression of tPA and uPA. First, terminally differentiated RGCs in the adult retina express low levels of tPA but not uPA.9 In contrast, RGC-5 cells differentiated after staurosporine treatment express elevated levels of tPA and uPA (present study). Second, although RGCs in the retina express low levels of tPA and RGC-5 cells in culture express low levels of uPA, at these low levels neither undergoes cell death; both these cell types undergo cell death only when uPA and tPA levels are elevated more than the physiological levels, as shown in this study and in our previous studies.8,9
These results raise some intriguing questions. First, what is the functional role of low levels of tPA in the retina? Previous studies have suggested that tPA proteolytically cleaves the NR1 subunit of the N-methyl D-aspartate (NMDA) receptor and induces calcium influx into neuronal cells.26 Based on these studies, we propose that, under physiological conditions, ganglion cells in the retina express low levels of tPA, perhaps to cleave glutamate receptors. Because visual processing requires rapid activation of glutamate receptors, RGCs may not have sufficient time to synthesize the tPA needed to process these receptors and, therefore, may express tPA constitutively.
Second, what is the functional role of uPA in undifferentiated RGC-5 cells? Although the physiological role of uPA in undifferentiated RGC-5 cells is unclear, previous studies on neuronal cells have reported that uPA, in a concentration-dependent manner, induces the release of calcium from internal stores.27 Therefore, we speculate that low levels of uPA may regulate physiological levels of calcium. It is also possible that undifferentiated RGC-5 cells do not express sufficient numbers of glutamate receptors for uPA to act on and to induce calcium influx. This is plausible because we have found that adding exogenous uPA or tPA (up to 5 μg/mL) to undifferentiated RGC-5 cells did not cause cell death (data not shown).
Third, why do differentiated RGC-5 cells secrete increased levels of tPA and uPA if these proteases eventually cause their death? Previous studies have suggested that tPA plays an important role in neurite outgrowth,17,18 formation of new synaptic connections,20 and activity-dependent synaptic plasticity in the central nervous system.22 Therefore, expression of low-level tPA might help neurite outgrowth under physiological conditions. However, when tPA is released in excess (under certain degenerative conditions, such as ischemia and excitotoxicity), it can cause cell death by processing glutamate receptors excessively, by increasing calcium influx, or by inducing additional proteases such as matrix metalloproteinases in astrocytes.28–30 Two recent studies reported that intravitreal injection of staurosporine induces the death of adult RGCs,31 and staurosporine-induced cell death can be attenuated by the broad-spectrum NMDA receptor antagonist MK801.32 Although it is unclear whether staurosporine-induced cell death in these studies was caused by increased expression of PAs, we speculate that PAs might play a role under these conditions.
In summary, we have provided evidence that staurosporine induces the differentiation of RGC-5 cells and elevates the levels of proteolytic activities of tPA and uPA and that these proteases, in turn, directly cause the death of RGC-5 cells. Our results also suggest that differentiated RGC-5 cells may be useful to screen pharmacologic agents that inhibit the proteolytic activities of plasminogen activators.
Acknowledgments
The authors thank Neeraj Agarwal (Department of Cell Biology and Genetics, North Texas Health Center) for providing RGC-5 cells.
Footnotes
Disclosure: R. Harvey, None; S.K. Chintala, None
Supported, in part, by National Institutes of Health project Grant EY13643, Vision Research Infrastructure Development Grant EY014803, and special research funds from Oakland University.
References
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA. Ganglion cell death in glaucoma: pathology recapitulates ontogeny. Aust NZ J Ophthalmol. 1995;23:85–91. doi: 10.1111/j.1442-9071.1995.tb00135.x. [DOI] [PubMed] [Google Scholar]
- 3.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 4.Osborne NN, Wood JP, Chidlow G, Bae JH, Melena J, Nash MS. Ganglion cell death in glaucoma: what do we really know? Br J Ophthalmol. 1999;83:980–986. doi: 10.1136/bjo.83.8.980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Osborne NN, Chidlow G, Layton CJ, Wood JP, Casson RJ, Melena J. Optic nerve and neuroprotection strategies. Eye. 2004;18:1075–1084. doi: 10.1038/sj.eye.6701588. [DOI] [PubMed] [Google Scholar]
- 6.Nickells RW. The molecular biology of retinal ganglion cell death: caveats and controversies. Brain Res Bull. 2004;62:439–446. doi: 10.1016/j.brainresbull.2003.07.007. [DOI] [PubMed] [Google Scholar]
- 7.Quigley HA, Nickells RW, Kerrigan LA, Pease ME, Thibault DJ, Zack DJ. Retinal ganglion cell death in experimental glaucoma and after axotomy occurs by apoptosis. Invest Ophthalmol Vis Sci. 1995;36:774–786. [PubMed] [Google Scholar]
- 8.Zhang X, Chaudhry A, Chintala SK. Inhibition of plasminogen activation protects against ganglion cell loss in a mouse model of retinal damage. Mol Vis. 2003;9:238–248. [PubMed] [Google Scholar]
- 9.Mali RS, Cheng M, Chintala SK. Plasminogen activators promote excitotoxicity-induced retinal damage. FASEB J. 2005;19:1280–1289. doi: 10.1096/fj.04-3403com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumada M, Niwa M, Wang X, et al. Endogenous tissue type plasminogen activator facilitates NMDA-induced retinal damage. Toxicol Appl Pharmacol. 2004;200:48–53. doi: 10.1016/j.taap.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Kumada M, Niwa M, Hara A, et al. Tissue type plasminogen activator facilitates NMDA-receptor-mediated retinal apoptosis through an independent fibrinolytic cascade. Invest Ophthalmol Vis Sci. 2005;46:1504–1507. doi: 10.1167/iovs.04-0595. [DOI] [PubMed] [Google Scholar]
- 12.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73:2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnamoorthy RR, Agarwal P, Prasanna G, et al. Characterization of a transformed rat retinal ganglion cell line. Brain Res Mol Brain Res. 2001;86:1–12. doi: 10.1016/s0169-328x(00)00224-2. [DOI] [PubMed] [Google Scholar]
- 14.Frassetto LJ, Schlieve CR, Lieven CJ, et al. Kinase-dependent differentiation of a retinal ganglion cell precursor. Invest Ophthalmol Vis Sci. 2006;47:427–438. doi: 10.1167/iovs.05-0340. [DOI] [PubMed] [Google Scholar]
- 15.Leprince P, Bonvoisin C, Rogister B, Mazy-Servais C, Moonen G. Protein kinase- and staurosporine-dependent induction of neurite outgrowth and plasminogen activator activity in PC12 cells. Biochem Pharmacol. 1996;52:1399–1405. doi: 10.1016/s0006-2952(96)00472-8. [DOI] [PubMed] [Google Scholar]
- 16.Charles I, Khalyfa A, Kumar DM, et al. Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci. 2005;46:1330–1338. doi: 10.1167/iovs.04-0363. [DOI] [PubMed] [Google Scholar]
- 17.Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988;11:541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- 18.Seeds NW, Haffke S, Christensen K, Schoonmaker J. Cerebellar granule cell migration involves proteolysis. Adv Exp Med Biol. 1990;265:169–178. doi: 10.1007/978-1-4757-5876-4_16. [DOI] [PubMed] [Google Scholar]
- 19.Sappino AP, Madani R, Huarte J, et al. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. doi: 10.1016/s0896-6273(00)80597-8. [DOI] [PubMed] [Google Scholar]
- 21.Davies BJ, Pickard BS, Steel M, Morris RG, Lathe R. Serine proteases in rodent hippocampus. J Biol Chem. 1998;273:23004–23011. doi: 10.1074/jbc.273.36.23004. [DOI] [PubMed] [Google Scholar]
- 22.Frey U, Muller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZL, Strickland S. Neuronal death in the hippocampus is promoted by plasmin-catalyzed degradation of laminin. Cell. 1997;91:917–925. doi: 10.1016/s0092-8674(00)80483-3. [DOI] [PubMed] [Google Scholar]
- 24.Tsirka SE, Rogove AD, Strickland S. Neuronal cell death and tPA. Nature. 1996;384:123–124. doi: 10.1038/384123b0. [DOI] [PubMed] [Google Scholar]
- 25.Tsirka SE, Rogove AD, Bugge TH, Degen JL, Strickland S. An extracellular proteolytic cascade promotes neuronal degeneration in the mouse hippocampus. J Neurosci. 1997;17:543–552. doi: 10.1523/JNEUROSCI.17-02-00543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nicole O, Docagne F, Ali C, et al. The proteolytic activity of tissue-plasminogen activator enhances NMDA receptor-mediated signaling. Nat Med. 2001;7:59–64. doi: 10.1038/83358. [DOI] [PubMed] [Google Scholar]
- 27.Christow SP, Bychkov R, Schroeder C, et al. Urokinase activates calcium-dependent potassium channels in U937 cells via calcium release from intracellular stores. Eur J Biochem. 1999;265:264–272. doi: 10.1046/j.1432-1327.1999.00729.x. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Cheng M, Chintala SK. Optic nerve ligation leads to astrocyte-associated matrix metalloproteinase-9 induction in the mouse retina. Neurosci Lett. 2004;356:140–144. doi: 10.1016/j.neulet.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 29.Wang X, Lee SR, Arai K, Tsuji K, Rebeck GW, Lo EH. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med. 2003;9:1313–1317. doi: 10.1038/nm926. [DOI] [PubMed] [Google Scholar]
- 30.Chintala SK. The emerging role of proteases in retinal ganglion cell death. Exp Eye Res. 2006;82:5–12. doi: 10.1016/j.exer.2005.07.013. [DOI] [PubMed] [Google Scholar]
- 31.Cordeiro MF, Guo L, Luong V, et al. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc Natl Acad Sci USA. 2004;101:13352–13356. doi: 10.1073/pnas.0405479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L, Salt TE, Maass A, et al. Assessment of neuroprotective effects of glutamate modulation on glaucoma-related retinal ganglion cell apoptosis in vivo. Invest Ophthalmol Vis Sci. 2006;47:626–633. doi: 10.1167/iovs.05-0754. [DOI] [PMC free article] [PubMed] [Google Scholar]


