Abstract
Background
Survivin is the smallest member of the inhibitor of apoptosis (IAP) gene family. Recently, the zebrafish survivin-1 gene has been cloned, showing remarkable sequence identity and similarity over the BIR domain compared with human and mouse survivin gene. Here we investigated the role of survivin in angiogenesis during zebrafish development. Morpholinos (MOs) targeting the 5' untranslated region (UTR) (SurUTR) and sequences flanking the initiation codon (SurATG) of zebrafish survivin-1 gene were injected into embryos at 1–4 cell stage. Vasculature was examined by microangiography and GFP expression in Tg(fli1:EGFP)y1 embryos. Results: In embryos co-injected with SurUTR and SurATG-MOs, vasculogenesis was intact but angiogenesis was markedly perturbed, especially in the inter-segmental vessels (ISV) and dorsal longitudinal anastomotic vessels (DLAV) of the trunk, the inner optic circle and optic veins of developing eyes and the sub-intestinal vessels. Apoptosis was increased, as shown by TUNEL staining and increase in caspase-3 activity. Efficacy of SurUTR and SurATG-MOs was demonstrated by translation inhibition of co-injected 5'UTR survivin:GFP plasmids. The phenotypes could be recapitulated by splice-site MO targeting the exon2-intron junction of survivin gene and rescued by survivin mRNA. Injection of human vascular endothelial growth factor (VEGF) protein induced ectopic angiogenesis and increased survivin expression, whereas treatment with a VEGF receptor inhibitor markedly reduced angiogenesis and suppressed survivin expression. Conclusion: Survivin is involved in angiogenesis during zebrafish development and may be under VEGF regulation.
Background
Survivin is the smallest member of the inhibitor of apoptosis (IAP) gene family containing a single Baculovirus IAP Repeat (BIR) domain and an extended -COOH terminal α-helical coiled coil [1]. Survivin is not expressed in most normal adult tissues but is highly expressed in solid and hematological malignancies, where it has been linked to increased angiogenesis and tumorigenesis [2,3]. During human and murine embryonic development, survivin is ubiquitously expressed [4]. However, homozygous knock-out of survivin in mouse ES cells results in disrupted microtubule formation and polyploidy as well as early embryonic fatality, precluding characterization of its functions during murine development [5]. As a result, the role of survivin during embryonic development remains unclear.
Recently, the zebrafish survivin-1 gene (abbreviated survivin) has been cloned, showing remarkable sequence identity and similarity over the BIR domain compared with human and mouse survivin gene [6]. Microarray analysis showed that survivin is significantly up-regulated in a zebrafish chordin morphant in which the intermediate cell mass (ICM, where vascular and primitive hematopoietic tissues arise) was expanded [7]. Here, we investigated if survivin plays a role in vascular formation during zebrafish embryonic development.
Results
Expression of survivin in zebrafish embryos
Whole-mount in-situ hybridization was performed to examine survivin mRNA expression in zebrafish embryos at 26 hpf. Survivin was detected diffusely throughout the developing brain and neural tube. It was also expressed at the vicinity of the axial vasculature from which the inter-segmental vessels arise (Figure 1a–b). This was further confirmed in histological sectioning in which the areas corresponding to the developing axial vasculature and neural tube showed increased expression relative to the adjacent tissues (Figure 1b, insert). Furthermore, double in-situ hybridization showed that survivin was expressed in the developing axial vasculature dorsal to the intermediate cell mass (ICM), where gene encoding for embryonic hemoglobin α was expressed. The pattern was remarkably similar to that of flk1, a VEGF receptor tyrosine kinase (Figure 1c–d).
Figure 1.

Whole-mount in-situ hybridization showing the expression of survivin in zebrafish embryos. (a, b): Survivin is expressed diffusely in the developing central nervous system (white arrows) and the axial vasculature (arrowheads) at 26 hpf. Similar expression patterns were seen at 56 hpf (not shown). (b, insert): Coronal section of stained embryos at 26 hpf showing preferential expression of survivin at the vicinity of the dorsal aorta and the developing neural tube (circled). (c, d): Double in-situ hybridization showing remarkably similar expression pattern of survivin (c) and flk1 (d) (blue, dark arrowheads) in relation to that of embryonic hemoglobin-α (brown, white arrowheads). Pictures are representative of at least three separate experiments. NT: Neural Tube; M: Myotome; N: Notochord; Y: Yolk sac extension.
Survivin morphants
The role of survivin during embryonic development was investigated by knocking-down its function using MOs. The phenotypic penetrance of survivin MOs was dose- and time-dependent. At 22 hpf, when injected with either 3 ng SurUTR or 3 ng SurATG-MOs (referred as SurUTRmo and SurATGmo embryos), most embryos had a relatively normal morphology (Figure 2a,c). However, at 48 hpf, 74.8 ± 7.3% and 72.0 ± 4.0% of embryos manifested "characteristic phenotypes" with reduced eye and head sizes and a mildly curved tail (Figure 2b,d). There was no overt tissue necrosis in these embryos. At 6 ng of either MOs, increasing numbers of embryos became severely deformed and died shortly after 48 hpf (Figure 2d, insert). Co-injecting SurATG + SurUTR-MOs (3 ng each) resulted in specific phenotypes in 79.4 ± 7.2% embryos without increase in toxicity or mortality as compared with 3 ng of either MO alone (Figure 2e). The combination regimen remained significantly less toxic than that of SurUTR-MO at 6 ng. In all subsequent experiments, SurATG and SurUTR-MOs were co-injected at 3 ng each (referred as SurUTR+ATGmo embryos). Only embryos with characteristic phenotypes were investigated while those which were severely deformed were excluded from analysis.
Figure 2.
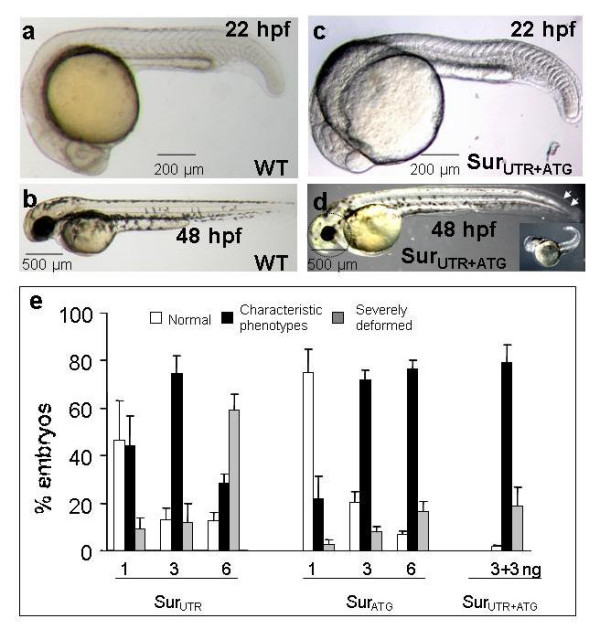
Effects of survivin knock-down on embryonic development. (a, b): Wild-type (WT) embryos injected with random sequence morpholino at 22 hpf (a) and 48 hpf (b). (c, d): Embryos injected with a combination of SurATG (3 ng) and SurUTR morpholinos (3 ng) (SurATG+UTR) at 1–4 cell stage. Noted that while there was no significant morphological changes at 22 hpf, most of the embryos injected with SurATG+UTR morpholinos at 48 hpf showed a "characteristic phenotype" with reduced head and eye size (circled) and a mildly curved tail (arrowheads). Similar phenotypes were also seen in embryos injected with either SurATG or SurUTR morpholinos at various doses but not in WT embryos injected with random sequence. Insert (d) showed a severe phenotype at 48 hpf characterized by severely deformed embryos which did not survive beyond 48 hpf. These embryos were not included in the analysis. Each picture is representative of at least three experiments. (e): The dose-dependence of either SurATG, SurUTR or SurATG+UTR morpholinos. Optimal response was observed when embryos were co-injected with 3 ng of each MO (SurUTR+ATG). Results were expressed as mean ± S.E.M. In each experiment, MOs at different doses were injected into the same batch of embryos and were scored at the same time. More than 40 embryos have been injected at each dosage.
Effects of survivin knock-down on angiogenesis
We have previously shown that survivin is significantly up-regulated in a zebrafish chordin morphant in which the ICM was expanded [7]. Therefore, we first examined the effects of survivin knock-down on vascular formation in Tg(fli1:EGFP)y1 embryos. In uninjected embryos, the axial circulation (AC), inter-segmental vessels (ISV), dorsal longitudinal anastomotic vessels (DLAV), vertebral and sub-intestinal vessels (SIV) were readily observable (Figure 3a,c). In SurUTR+ATGmo embryos, the dorsal aorta and posterior cardinal vein were patent, indicative of intact vasculogenesis (see additional file 1: Wild-type embryos and file 2: Survivin morphants). However, the development of vertebral and ISV was perturbed with defective or total absence of sprouting as well as failure to form the DLAV and SIV (Figure 3b,d). These defects were seen in all 54 SurUTR+ATGmo embryos observed (n = 3 separate experiments) with the characteristic phenotypes. The results were confirmed using microangiography in which defective ISV sprouting and failure to form the DLAV, as well as defective inner optic circle (IOC) and optic veins (OV) of the developing eyes were seen in the SurUTR+ATGmo embryos (Figure 3e–h). Similar patterns of angiogenesis defects were observed when either SurUTR or SurATG morpholinos were injected (data not shown).
Figure 3.

Effects of survivin knock-down on angiogenesis and circulation. (a, b): Confocal microscopy of Tg(fli1:EGFP)y1 embryos either uninjected (a) or injected with SurUTR+ATG morpholinos (b). Noted the aberrant sprouting of the inter-segmental vessels (ISV) (arrowheads), the absence of vertebral arteries (arrows) and the failure to form the dorsal anastomotic vessels (DLAV) in the SurUTR+ATGMO embryos. AC: Axial circulation. Noted that the dorsal aorta and posterior cardinal vein in the axial circulation could not be distinguished based on the resolution provided. (c, d): Fluorescent images in Tg(fli1:EGFP)y1 embryos showing failure to develop the sub-intestinal vessels (SIV) in SurUTR+ATGMO embryos. (e-h): Microangiographic pictures in uninjected (e, g) and SurUTR+ATGMO embryos (f, h) showing defective vasculatures in ISV, DLAV, optical veins (OV) and inner optic circle (IOC). N: Notochord; AC: Axial circulation. (i, j): Whole-mount TUNEL assay in embryos injected with random sequence MO (i) and SurUTR+ATG-MO (j) showing positive staining in the area of developing neural tube and brain (white arrows) as well as at the vicinity of the axial circulation (white arrowheads) in the SurUTR+ATGMO embryos. N: Notochord; Y: Yolk sac extension. Embryos were examined at 48 hpf except (c) & (d) which were examined at 96 hpf. More than 20 embryos have been examined in each experiment.
Effects of survivin MOs on apoptosis as shown by TUNEL and caspase-3 activity
As a member of the IAP family, survivin has been shown to inhibit apoptosis by regulating caspase activity [1,2]. Therefore; we investigated if there was increased apoptosis in the SurUTR+ATGmo embryos as measured by TUNEL assay. At both 24 and 48 hpf, increased TUNEL staining was detected in the developing neural tube and the brain (not shown), with significant, albeit weaker, staining at the vicinity of the axial vasculature (Figure 3i,j). The increased apoptosis was further confirmed by specific caspase-3 activity which was significantly increased in 48 hpf SurUTR+ATGmo embryos (299.1 ± 8.3 arbitrary units) compared with control embryos injected with a random sequence MO at 6 ng (103.0 ± 2.3 arbitrary units, n = 3 experiments using 240 embryos, p < 0.05).
Specificity of survivin knock-down
To further demonstrate the efficacy of SurUTR and SurATG MO binding to survivin mRNA, embryos were co-injected with a 5'UTR survivin:GFP plasmid (50 pg) and SurUTR+ATG-MOs (3 ng each). Injecting the plasmid alone lead to GFP expression in 79.7 ± 9.4% embryos (Figure 4a,c). Co-injection of the plasmid with SurUTR+ATG-MOs completely abolished protein translation and hence GFP expression in all embryos tested (Figure 4b,d). A splice site MO (SurSS-MO (12 ng)) not only induced similar morphological changes as in SurUTR+ATGmo embryos (smaller head and eye size and mildly curved tail) but also induced defective angiogenesis as shown in Tg(fli1:EGFP)y1 embryos (61.7%, n = 3 experiments using 159 embryos) (Figure 4e–f). Angiogenesis defects were seen in ISV as well as OV/IOC of the developing eyes (not shown). A relatively high dose of MO (12 ng) was used as lower doses produced less phenotypic penetrance and at 12 ng, there was no excessive mortality. In the SurSSMO embryos, RT-PCR confirmed defective splicing of part of the intron, as shown by a larger PCR transcript which was verified by bi-directional DNA sequencing (Figure 4g,h). Whether defective splicing could be induced by lower doses of this MO has not been examined. Finally, defective sprouting or failure to form the DLAV occurred in all SurUTR+ATGmo embryos and co-injecting survivin mRNA (30 pg) with SurUTR+ATG-MOs rescued the vascular defect in 47 out of 58 embryos in three separate experiments (81%) (Figure 4i–l).
Figure 4.
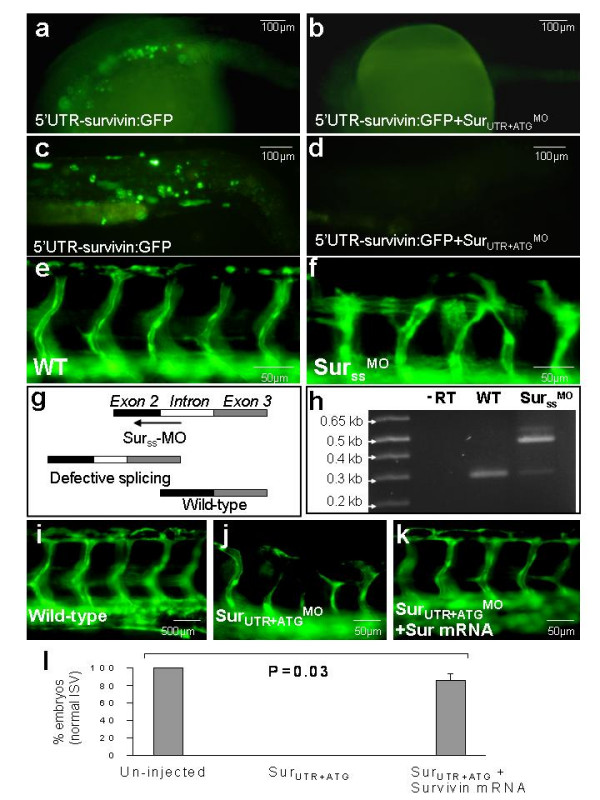
Effect of survivin knock-down was gene-specific. (a-d): Injection of 5'UTR-survivin:GFP plasmids gave rise to green fluorescence in a mosaic pattern in 79.7 ± 9.4% (a, c) which was totally abolished by co-injection with SurUTR+ATG-MO (b, d). (e): Uninjected Tg(fli1:EGFP)y1 embryos at 48 hpf. (f): Defective sprouting of inter-segmental vessels, similar to those seen in SurUTR+ATGMO embryos, could be recapitulated by injecting embryos with survivin morpholino targeting the splice-site junction (g). (h): Molecular targeting was confirmed using RT-PCR showing survivin gene in injected embryos contained a larger transcript compared with uninjected ones. (i-k): Defective angiogenesis in SurUTR+ATGMO embryos could be rescued by co-injecting with survivin mRNA. (i): Uninjected embryos. (j): SurUTR+ATGMO embryos. (k): SurUTR+ATGMO embryos co-injected with survivin mRNA. (i): Histogram showing average number of embryos with normal inter-segmental vessels (ISV) in three separate experiments. All embryos were oriented anterior (left) to posterior (right).
Effects of VEGF on survivin expression
VEGF plays an important role in angiogenesis during zebrafish embryonic development [8]. In-vitro studies have shown that survivin mediates the proliferative and anti-apoptotic effects of VEGF in endothelial cells [9]. Therefore; we investigated if survivin expression during embryogenesis is regulated by VEGF. Exogenous human VEGF protein (2 ng) was injected into zebrafish embryos at one-cell stage [10]. Angiogenesis was examined in the sub-intestinal vessels at 96 hpf, where the vasculature was well-developed and any ectopic structures could be readily detectable. In 78 out of 110 embryos (70%) (from three separate experiments), VEGF induces ectopic angiogenesis which was associated with a significant up-regulation of survivin mRNA expression (Figure 5a–b,f). We also incubated embryos with a VEGF receptor inhibitor (VEGFTKR) at one-cell stage. VEGFTKR (25 μmol/L) induced defective angiogenesis in all treated embryos at 48 hpf (Figure 5c–d) and could not be rescued by survivin mRNA injection (30 pg) (Figure 5e). Survivin mRNA expression was significantly down-regulated in these embryos (Figure 5f).
Figure 5.
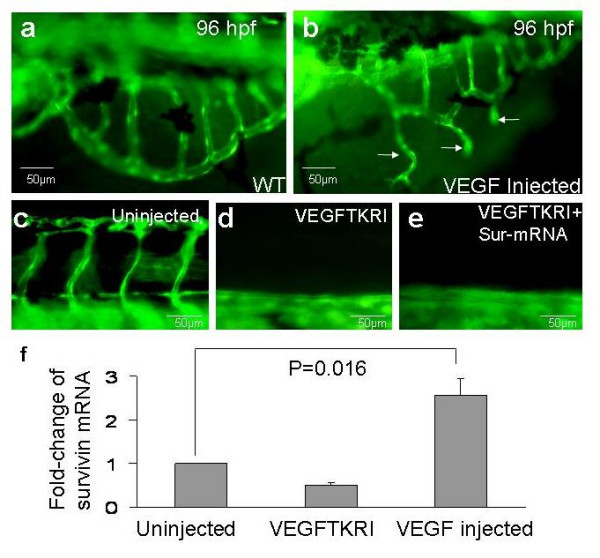
Regulation of survivin expression by vascular endothelial growth factor (VEGF) at 96 hpf. (a): Sub-intestinal vessels in uninjected Tg(fli1:EGFP)y1 embryos. (b): Injection of human VEGF (2 ng) gave rise to ectopic angiogenesis (arrows). There was no observable ectopic angiogenesis in the ISV (c): Axial and inter-segmental vessels in untreated Tg(fli1:EGFP)y1 embryos. (d): Tg(fli1:EGFP)y1 embryos treated with VEGF tyrosine kinase receptor inhibitor (VEGFTKRI, 25 μmol/L) showing defective sprouting of inter-segmental vessels. (e): Injection of survivin mRNA did not reverse the defects seen in VEGFTKRI treated embryos. (f): Histogram showing the average survivin mRNA expression (expressed in fold-change) in untreated and VEGFTKRI treated embryos as well as in embryos injected with human VEGF. Results expressed in mean ± S.E.M. (n = 3 experiments using at least 20 embryos per experiments). When the three groups of data were compared using Kruskal-Wallis Test, p-value = 0.016. When the data of uninjected vs VEGFTKRI treated embryos were compared using Mann-Whitney U Test, p-value = 0.037.
Discussion
In this study, we demonstrated that knock-down of survivin with MOs gives rise to embryos with reduced eye and head sizes and a mildly curved tail. Similar phenotypic changes have been described in a survivin mutant generated in a retrovirus insertional mutagenesis screen [11]. The survivin morphants had defective angiogenesis but vasculogenesis, i.e. formation of axial vasculature, was not affected at the doses of MOs tested. Development delay in these morphants was not observed, as shown by the normal onset and pattern of pigmentation and heart beat (data not shown). Our results corroborate with in-vitro studies showing that survivin is important for the maintenance of proliferation and survival on endothelial cells [9,12]. In addition, our data provided new information on the role of survivin during embryonic development.
In-vitro and tumorigenesis studies have shown that survivin mediates the angiogenic effects of VEGF [9,12,13]. In zebrafish embryos, VEGF signaling is important for angiogenesis. In particular, mutants defective in a zebrafish orthologue of flk1 (a VEGF-receptor tyrosine kinase), the schwentine [14], and in phospholipase Cγ (a tyrosine kinase mediating effects of VEGFR), the y10 [15], exhibit specific defects in angiogenesis. MO targeting of VEGF results in defective circulation in the head, axial and inter-segmental vasculature in a dose-dependent fashion [8]. In this study, VEGF induces ectopic angiogenesis and up-regulates survivin mRNA expression, suggesting that survivin may mediate the angiogenic effect of VEGF. The link between VEGF and survivin during zebrafish angiogenesis has not been examined but may involve PKB/Akt signaling as reported in human endothelial cell lines [16]. Intriguingly, co-injecting embryos with survivin mRNA could only rescue the vascular defects seen in SurUTR+ATGMO embryos but not in embryos treated with a VEGF receptor inhibitor. Therefore, additional downstream mediators may be involved in the angiogenic effects of VEGF [17]. Reversely, whether VEGF can rescue the angiogenesis defects in SurUTR+ATGMO embryos has not been examined. Perturbation of VEGF signaling may also result in changes in blood vessel synthesis and the observed changes in survivin mRNA may reflect changes in endothelial cell number rather than a direct mechanistic link to VEGF signaling. This issue would have to be evaluated in future study.
Both human and murine studies have demonstrated that survivin is involved in haematopoietic stem and progenitor cell proliferation [18,19]. However, in the present study, early specification of hematopoietic progenitors in the SurUTR+ATGMO embryos was not affected, as shown by the normal expression of genes encoding for hematopoietic transcription factors and embryonic hemoglobins, as well as the normal distribution of gata1+ population in Tg(gata1:GFP) embryos at 18 hpf, before the onset of functional circulation (data not shown).
That the targeting of the survivin MOs was specific was shown using several control studies. First, the phenotypic changes seen in SurUTR+ATGMO embryos were similar to those observed in survivin mutants generated by retrovirus insertional mutagenesis screening [11]. Indeed, it would be valuable to examine this mutant for similar defects in angiogenesis. Second, co-injecting SurUTR+ATG-MO with a 5'UTR-survivin:GFP plasmid inhibited translation and hence green fluorescence induced by the latter in all embryos, proving efficacious binding of SurUTR+ATG-MO to the 5'UTR-survivin mRNA. Third, the angiogenesis defects of ISV induced by SurUTR+ATG-MO could be rescued by survivin mRNA. Whether the defects in SIV and OV/IOC were similarly reversed and whether injection of survivin mRNA alone would induce angiogenesis defects would have to be further examined. Finally a splice-site morpholino recapitulated the phenotypes seen with SurUTR+ATG-MO. Therefore, the angiogenesis defects in SurUTR+ATGMO embryos represent a specific phenotype due to knock-down of survivin function in zebrafish embryos.
In human, murine [4] as well as Xenopus embryos [20], survivin is ubiquitously expressed. These observations, together with the fact that the developing head and eye of the SurUTR+ATGMO embryos were reduced in size have raised a concern whether the angiogenesis defects in these embryos might be caused by a general developmental defect and cell death, rather than a specific requirement for survivin function. However, in zebrafish embryos, survivin is more robustly expressed in the axial vasculature as well as the developing brain and neural tube. This observation has been confirmed by histological sectioning as well as double in-situ hybridization in which the pattern of survivin expression showed remarkable similarity to that of flk1, a marker of vascular endothelium. We cannot exclude a low level but diffuse expression of survivin in adjacent tissues but this should not negate the specific role of survivin during angiogenesis. First, we have confined our examination to the SurUTR+ATGMO embryos with characteristic phenotypes and none of them showed overt tissue necrosis at 48 hpf. The 20% embryos with severe deformity have been excluded from analysis. Second, we demonstrated that embryonic development is not overtly delayed in the SurUTR+ATGMO embryos as shown by the normal onset and pattern of pigmentation and heart beat (data not shown). Finally, in the present study, survivin expression appeared to be under VEGF regulation, providing a possible link between VEGF and embryonic angiogenesis. Our findings were also consistent with those by Pasquier et al. [20] in which over-expression of survivin in Xenopus embryos induces endothelial cell proliferation in-vivo.
Several observations in this study have remained unexplained. For instance, we did not observe a direct causal link between increased apoptosis and the angiogenesis defect in the SurUTR+ATGMO embryos. Apoptosis was detectable not only in the axial vasculature, but also in the developing brain and neural tube of the SurUTR+ATGMO embryos. Both in-vivo and in-vitro studies have demonstrated that in addition to its anti-apoptotic function, survivin plays an important role in the regulation of cellular proliferation and cytokinesis [1,2]. Recent study in Xenopus embryos also showed that survivin expression induces endothelial cell proliferation independent of apoptosis [20]. Therefore, the relative modest TUNEL staining in the axial vasculature did not preclude the role of survivin in angiogenesis. It is also possible that survivin plays a non-cell autonomous role in the angiogenesis process. Childs et al. (2002) [21] demonstrated in zebrafish embryos the migration of angioblasts from the aorta to the dorsal aspect of the neural tube and to the inter-phase between notochord and the somites, where they develop into DLAV and ISV. Therefore, vascular patterning may depend on signaling cues that direct the site of angiogenesis sprouts. Whether the occurrence of apoptosis in the developing neural tube might have perturbed these signals hence the formation of DLAV and ISV would have to be carefully examined. The proposition may also explain the lack of robust expression at the site of ISV and DLAV in wild-type embryos. Furthermore, although survivin is expressed robustly in the axial vasculature, concomitant expression was noted in the developing central nervous system. The expression of survivin within these structures needs to be defined in future study. Moreover, the developing eye and head structures in the survivin morphants are generally smaller. Whether this reflected changes secondary to defective angiogenesis or alternative functions of survivin during development have not been elucidated. Finally, survivin gene in zebrafish has undergone duplication during evolution [22] and the function of the duplicated gene would have to be further investigated. Notwithstanding these limitations, our data still supported the proposition that survivin is involved in the regulation of angiogenesis during zebrafish development.
Survivin is strongly expressed in both solid organ and hematological malignancies where it is associated with treatment failure and a poor prognosis [2,3]. Loss of function studies have also demonstrated that survivin expression is linked to angiogenesis and tumorigenesis in gastric and colonic cancers and has become a potential target for anti-cancer therapy [23,24]. Our observation that survivin regulates angiogenesis in zebrafish embryos highlights the relevance of using zebrafish embryos in the screening for survivin-based anti-cancer agents.
In summary, we demonstrate that survivin plays an important role in angiogenesis during embryonic development and may be one of the down-stream effectors of VEGF signaling. Early hematopoiesis was not affected but the role of survivin during late hematopoiesis remains to be determined.
Methods
Zebrafish and morpholinos
Wild-type zebrafish (Danio rerio) were obtained from local aquarium and were maintained and raised under standard conditions at 28°C. Transgenic Tg(fli1:EGFP)y1 embryos were used to track endothelial cell populations. Anti-sense morpholinos (MO) (Gene-Tools, OR, USA) were designed to target the 5'untranslated region (UTR) or sequences flanking and including the initiation codon (ATG) of the zebrafish survivin gene. A splice-site (SS) MO was designed to target the exon2-intron junction of the survivin gene (SurSS-MO). A random sequence MO was used as a control as described previously (Table 1). Procedures for micro-injection, whole mount in-situ hybridization, microangiography, TUNEL and caspase-3 activity assays have been described previously [7,25,26].
Table 1.
Sequences of oligos used.
| Oligo | Sequence |
| Morpholinos | |
| SurATG | TGC AAG ATC CAT TTT GTG GGA GGT T |
| SurUTR | GTG GAA ATT AAA CAA AAG ACA ACC G |
| SurSS | AGA CAC GGA CTC ACT CAG GGT CAT C |
| Random Sequence | CCT CTT ACC TCA GTT ACA ATT TAT A |
| Primers for the cloning of survivin mRNA in riboprobe synthesis | |
| ZF Surf | CGG ATT TAT CTC GGT TGT CTT T |
| ZF Surr | CAA CTT TCA CAA GTG ACA GAA CAC |
| Primers for the cloning of survivin UTR for 5'UTR survivin-GFP construct synthesis | |
| ZF SurUTRf | GCG GAT TTA TCT CGG TTG TCT |
| ZF SurUTRr | CTT CCT CCC CCA TCG CAG TCT GG |
| Primers for RT-PCR for survivin mRNA in splice-site morpholino study | |
| ZF Surf | CAA CCT CCC ACA AAA TGG AT |
| ZF Surr | GTC CAC AGT CTT CTT CAG CA |
| Primers for the cloning of survivin mRNA in rescue experiments | |
| ZF Surf | AAT CAA CAA GCA AGCGAG AC |
| ZF Surr | CAA TTT ATT AAG CCC GAA TGC |
| Primers for real-time quantitative RT-PCR for survivin mRNA | |
| ZF Surf | CAC TCC AGA AAA CAT GGC TAA A |
| ZF Surr | CCA TCC TTC CAG CTC TTT CA |
Double in-situ hybridization
Wild-type (WT) embryos at 24 hpf were fixed with 4% paraformaldehyde (PFA) and dehydrated. After stepwise re-hydration, the embryos were incubated in pre-hybridization buffer (50% formamide, 5 × SSC, 50 μg/ml heparin, 0.1% Tween20, 5 mg/ml rRNA in phosphate-buffered saline, PBS) at 65°C followed by overnight incubation with digoxigenin (DIG)- (either flk-1 or survivin-1) and fluorescein-labeled riboprobe (α-embryonic globin) at 65°C. The embryos were washed and incubated with alkaline phosphatase (AP) conjugated anti-DIG antibody (Roche Molecular Biochemicals, Mannheim, Germany) for overnight at 4°C. Blue color was developed using NBT/BCIP (Roche Molecular Biochemicals, Mannheim, Germany) as substrate and the reaction was stopped with 0.5 mM EDTA in PBT. AP was destroyed by washing the stained embryos with 0.1 M glycine-HCl, pH 2.2 in PBT for 10 min twice. Background staining was removed by washing the embryos in absolute ethanol with continuous monitoring. After re-hydration to PBT, embryos were incubated with AP conjugated anti-fluorescein antibody (Roche Molecular Biochemicals, Mannheim, Germany) for overnight at 4°C and red color was developed using INT/BCIP (Roche Molecular Biochemicals, Mannheim, Germany) as substrate.
Synthesis of anti-sense mRNA riboprobe for survivin
The full length sequence of zebrafish survivin including the 3' UTR was amplified by PCR (Table 1) from cDNA of 24 hpf embryos and subcloned into pGem-T vector (pGEM-T Vector Systems, Promega, Madison, WI, USA). A 623 bp anti-sense survivin mRNA riboprobe was synthesized from linearized vector containing the insert. A digoxigenin labeled mRNA probe was synthesized by SP6 RNA polymerase according the manufacturer's protocols (Roche Applied Science, Indianapolis, IN, USA). The size and integrity of the synthesized riboprobe was confirmed by RNA formaldehyde gel electrophoresis. Histological assessment of stained embryos was performed on 5–7-μm paraffin sections.
Construction of 5'UTR-survivin:GFP plasmids
The 5'UTR of survivin, including the target sequences of SurUTR-MO and SurATG-MO, were amplified from 24 hpf wild-type embryo cDNA (Table 1). PCR products were gel purified and cloned in frame and immediately upstream of the GFP coding sequence into vector pcDNA3.1/CT-GFP-TOPO (Invitrogen, Carlsbad, CA, USA) and transformed into chemically competent E. coli TOP10 (Invitrogen, Carlsbad, CA, USA). Plasmids containing the 5'UTR-survivin:GFP fusion sequence were isolated and the sequence of the DNA inserts verified using the GFP reverse primer (5'-GGG TAA GCT TTC CGT ATG TAG C-3').
Preparation of survivin mRNA for rescue experiments
The complete coding sequence of survivin was TA-cloned into pGEM-T vector (pGEM-T Vector Systems, Promega, Madison, WI, USA) and the orientation of the insert confirmed by PCR (Table 1). mRNA transcripts were synthesized from the T7 promoter of the Sal I digested pGEMT-Sur sequence using the mMessage mMachine Kit (Ambion, Austin, TX, USA).
Treatment of embryos with VEGF receptor tyrosine kinase inhibitors
Embryos were treated with an inhibitor of vascular endothelial growth factor receptor tyrosine kinase (VEGFRTK inhibitors, Calbiochem, EMD Bioscience, CA, USA). The embryos were incubated in inhibitor solution at 25 μmol/L (stock solution in DMSO at 10 mmol/L) from one-cell stage onwards. They were dechorionated at 24 hpf with continuous exposure to inhibitors until 48 hpf. Control experiments were set up from the same clutches of embryos and were exposed to equal volume of DMSO for comparison.
Vascular endothelial growth factor (VEGF) injection
Human VEGF protein (BD Bioscience, Bedford, MA, USA) was prepared in 1 mg/mL in water. Embryos at 1–4 cell stage were injected with VEGF (2 ng) into the yolk sac and its effect on angiogenesis was examined at 96 hpf.
Real-time quantitative RT-PCR (Q-PCR)
cDNA from 48 and 96 hpf embryos were reverse transcribed from RNA and Q-PCR for survivin was performed using the SYBR green PCR master mix (Applied Biosystems, Foster City, CA, USA). Expression level was presented as fold-change calculated using the comparative CT method as described [27] with β-actin as the internal reference. Primer sequences for Q-PCR were shown in Table 1.
Statistical analysis
Results were expressed as mean ± SEM unless otherwise stated. Comparisons between groups of data were evaluated by Mann-Whitney U and Kruskal-Wallis Test where appropriate. P-value of less than 0.05 was considered statistically significant.
Authors' contributions
ACHM carried out the microinjection and molecular studies and wrote the manuscript. RL carried out the microinjection in some experiments. PKC carried out the confocal microscopy. JL and LC performed the histological sectioning of embryos. AM, CV and RL participated in the design of the study. AYHL conceived of the study, and participated in its design and coordination and wrote the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Blood circulation in wild-type embryos. In wild-type embryos, normal axial circulation is observed as well as circulation in ISV and DLAV.
Blood circulation in survivin morphants. In survivin morphants, normal axial circulation is observed but circulation in ISV and DLAV was absent.
Acknowledgments
Acknowledgements
We would like to thank Dr. Stephen C Ekker, Department of Genetics, Cell Biology and Development, University of Minnesota, MN, USA for his helpful discussion and comments on the manuscript. We also thanked Dr. S.H. Cheng and the Confocal Facility in City University of Hong Kong for focal microscopy. Thanks are extended to Jessie Fu, Babs Kwok and Tommy Kwan for performing some of the microinjection experiments and to Mr. Howard Chow for part of the molecular studies.
This work was supported by RGC grant (HKU 7488/04M and 7520/06M) and small project funding from CRCG (HKU).
Contributor Information
Alvin CH Ma, Email: h0025231@hkusua.hku.hk.
Rachel Lin, Email: rachellin@hku.hk.
Po-Kwok Chan, Email: bhechan@cityu.edu.hk.
Joseph CK Leung, Email: jckleung@hku.hk.
Loretta YY Chan, Email: lyychan@hku.hk.
Anming Meng, Email: mengam@mail.tsinghua.edu.cn.
Catherine M Verfaillie, Email: verfa001@umn.edu.
Raymond Liang, Email: rliang@hku.hk.
Anskar YH Leung, Email: ayhleung@hku.hk.
References
- Altieri DC. Molecular circuits of apoptosis regulation and cell division control: The survivin paradigm. J Cell Biochem. 2004;92:656–663. doi: 10.1002/jcb.20140. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nature Reviews Cancer. 2003;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Graaf AO, de Witte T, Jansen JH. Inhibitor of apoptosis proteins: new therapeutic targets in hematological cancer? Leukemia. 2004;18:1751–1759. doi: 10.1038/sj.leu.2403493. [DOI] [PubMed] [Google Scholar]
- Adida C, Crotty PL, Berrebi MD, Diebold J, Altieri DC. Developmentally regulated expression of the novel cancer anti-apoptosis gene survivin in human and mouse differentiation. Am J Pathol. 1998;152:43–49. [PMC free article] [PubMed] [Google Scholar]
- Uren AG, Wong L, Pakusch M, et al. Survivin and the inner centromere protein INCENP show similar cell-cycle localization and gene knockout phenotype. Curr Biol. 2000;10:1319–28. doi: 10.1016/S0960-9822(00)00769-7. [DOI] [PubMed] [Google Scholar]
- Inohara N, Nuñez G. Genes with homology to mammalian apoptosis regulators identified in zebrafish. Cell Death Diff. 2000;7:509–510. doi: 10.1038/sj.cdd.4400679. [DOI] [PubMed] [Google Scholar]
- Leung AY, Mendenhall EM, Kwan TT, et al. Characterization of expanded intermediate cell mass in zebrafish chordin morphant embryos. Dev Biol. 2005;277:235–54. doi: 10.1016/j.ydbio.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Larson J, Ekker SC. Distinct requirements for zebrafish angiogenesis revealed by a VEGF-A morphant. Yeast. 2000;17:294–301. doi: 10.1002/1097-0061(200012)17:4<294::AID-YEA54>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesri M, Morales-Ruiz M, Ackermann EJ, et al. Suppression of vascular endothelial growth factor-mediated endothelial cell protection by survivin targeting. Am J Pathol. 2001;158:1757–1765. doi: 10.1016/S0002-9440(10)64131-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbedzija GN, Flynn E, Willett CE. Zebrafish angiogenesis: A new model for drug screening. Angiogenesis. 1999;3:353–359. doi: 10.1023/A:1026598300052. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Nissen RM, Sun Z, Swindell EC, Farrington S, Hopkins N. Identification of 315 genes essential for early zebrafish development. Proc Natl Acad Sci USA. 2004;101:12792–7. doi: 10.1073/pnas.0403929101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J, Rak J, Sheehan C, et al. Marked induction of the IAP family antiapoptotic proteins survivin and XIAP by VEGF in vascular endothelial cells. Biochem Biophys Res Commun. 1999;264:781–788. doi: 10.1006/bbrc.1999.1589. [DOI] [PubMed] [Google Scholar]
- Beierle EA, Nagaram A, Dai W, Iyenger M, Chen MK. VEGF-mediated survivin expression in neuroblastoma cells. J Surg Res. 2005;127:21–28. doi: 10.1016/j.jss.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Habeck H, Odenthal J, Walderich B, Maischein HM, Consortium TS, Schulte-Merker S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12:1405–1412. doi: 10.1016/S0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Mugford JW, Diamond BA, Weinstein BM. Phospholipase C gamma-1 is required downstream of vascular endothelial growth factor during arterial development. Genes Dev. 2003;17:1346–51. doi: 10.1101/gad.1072203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran J, Master Z, Rak J, Dumont DJ, Kerbel RS. A role for survivin in chemoresistance of endothelial cells mediated by VEGF. Proc Natl Acad Sci. 2002;99:4349–4354. doi: 10.1073/pnas.072586399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung T, Chen H, Stauffer AM, et al. Zebrafish G protein {gamma}2 is required for VEGF signaling during angiogenesis. Blood. 2006 Mar 14. [DOI] [PMC free article] [PubMed]
- Fukuda S, Pelus LM. Regulation of the inhibitor-of-apoptosis family member survivin in normal cord blood and bone marrow CD34+ cells by hematopoietic growth factors: implication of survivin expression in normal hematopoiesis. Blood. 2001;98:2091–2100. doi: 10.1182/blood.V98.7.2091. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Foster RG, Porter SB, Pelus LM. The antiapoptotic protein survivin is associated with cell cycle entry of normal cord blood CD34+ cells and modulates cell cycle and proliferation of mouse hematopoietic progenitor cells. Blood. 2002;100:2463–2471. doi: 10.1182/blood.V100.7.2463. [DOI] [PubMed] [Google Scholar]
- Pasquier DP, Phung AC, Ymlahi-Ouazzani Q, et al. Survivin increased vascular development during Xenopus ontogenesis. Differentiation. 2006;74:244–253. doi: 10.1111/j.1432-0436.2006.00073.x. [DOI] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryos. Development. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Force A, Lynch M, Pickett FB, Amores A, Yan YL, Postlethwait J. Preservation of duplicate genes by complementary, degenerative mutations. Genetics. 1999;151:1531–1545. doi: 10.1093/genetics/151.4.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu SP, Jiang XH, Lin MC, et al. Suppression of survivin expression inhibits in vivo tumorigenicity and angiogenesis in gastric cancer. Cancer Research. 2003;63:7724–7732. [PubMed] [Google Scholar]
- Tu SP, Cui JT, Liston P, et al. Gene therapy for colon cancer by adeno-associated viral vector-mediated transfer of survivin Cys84Ala mutant. Gastroenterology. 2005;128:361–75. doi: 10.1053/j.gastro.2004.11.058. [DOI] [PubMed] [Google Scholar]
- Kwan TT, Liang R, Verfaillie CM, et al. Regulation of primitive hematopoiesis in zebrafish embryos by the death receptor gene. Expt Haematol. 2006;34:27–34. doi: 10.1016/j.exphem.2005.09.017. [DOI] [PubMed] [Google Scholar]
- Chen E, Hermanson S, Ekker SC. Syndecan-2 is essential for angiogenic sprouting during zebrafish development. Blood. 2004;103:1710–19. doi: 10.1182/blood-2003-06-1783. [DOI] [PubMed] [Google Scholar]
- Ma AC, Liang R, Leung AY. The role of phospholipase C gamma 1 in primitive hematopoiesis during zebrafish development. Exp Hematol. 2007 doi: 10.1016/j.exphem.2006.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Blood circulation in wild-type embryos. In wild-type embryos, normal axial circulation is observed as well as circulation in ISV and DLAV.
Blood circulation in survivin morphants. In survivin morphants, normal axial circulation is observed but circulation in ISV and DLAV was absent.


