Abstract
Aim
Cytochrome P450 2E1 (CYP2E1) is thought to activate a number of protoxins, and has been implicated in the development of liver disease. Increased hepatic expression of CYP2E1 occurs in rat models of diabetes but it is unclear whether human diabetics display a similar up-regulation. This study was designed to test the hypothesis that human diabetics experience enhanced CYP2E1 expression.
Methods
The pharmacokinetics of a single dose of chlorzoxazone (500 mg), used as an index of hepatic CYP2E1 activity, was determined in healthy subjects (n = 10), volunteers with Type I (n = 13), and Type II (n = 8) diabetes mellitus. Chlorzoxazone and 6-hydroxychlorzoxazone in serum and urine were analysed by high-performance liquid chromatography. The expression of CYP2E1 mRNA in peripheral blood mononuclear cells was quantified by reverse transcriptase-polymerase chain reaction.
Results
The mean ± s.d. (90% confidence interval of the difference) chlorzoxazone area under the plasma concentration-time curve was significantly (P ≤ 0.05) reduced in obese Type II diabetics (15.7 ± 11.3 µg h ml−1; 9, 22) compared with healthy subjects (43.5 ± 16.9 µg h ml−1; 16, 40) and Type I diabetics (32.8 ± 9.2 µg h ml−1; 9, 25). There was a significant two-fold increase in the oral clearance of chlorzoxazone in obese Type II diabetics compared with healthy volunteers and Type I diabetics. The protein binding of chlorzoxazone was not significantly different between the three groups. In contrast, Type 1 diabetics and healthy volunteers demonstrated no difference in the oral clearance of chlorzoxazone. The urinary recovery of 6-hydroxychlorzoxazone as a percentage of the administered dose was not different between healthy, Type I and obese Type II diabetics. The elimination half-life of chlorzoxazone did not differ between the three groups. CYP2E1 mRNA was significantly elevated in Type I and obese Type II diabetics compared with healthy volunteers. The oral clearance of chlorzoxazone, elimination half-life, Tmax, and Cmax were not significantly influenced by weight, body mass index, serum glucose, serum cholesterol, or glycosylated haemoglobin.
Conclusions
There was a marked increase in hepatic CYP2E1 activity in obese Type II diabetics as assessed by chlorzoxazone disposition. Increased expression of CYP2E1 mRNA in peripheral blood mononuclear cells was found in both types of diabetes mellitus. Adverse hepatic events associated with Type II diabetes may be in part a result of enhanced CYP2E1 expression and activity.
Keywords: chlorzoxazone, CYP2E1, diabetes, insulin
Introduction
The cytochromes P450 (CYP) are a superfamily of haemoproteins that mediate the biotransformation of endogenous and exogenous compounds [1]. In humans, three cytochrome P450 subfamilies composed of 11 genes are responsible for the metabolism of most drugs [2]. CYP2E1 is the classical ethanol-inducible CYP, and this is highly significant because it has been shown to catalyse the bioactivation of several procarcinogens and protoxins including N-nitrosodimethylamine, benzene and N-alkylformamides [3, 4]. In addition to oxidizing ethanol, human CYP2E1 has also been shown to metabolize drugs such as chlorzoxazone, acetaminophen, and the volatile anaesthetics (enflurane, sevoflurane, methoxyflurane and isoflurane) [5, 6].
In addition to its role as a xenobiotic-metabolizing enzyme, CYP2E1 expression has been linked to the generation of specific pathological conditions, including alcoholic and nonalcoholic liver disease [7, 8]. This link stems from the unusually high capacity of CYP2E1 to generate free radicals due to a low degree of coupling between enzyme turnover and substrate binding. The free radicals produced by CYP2E1 are thought to result in lipid peroxidation and thus contribute to liver disease. Diabetes is commonly associated with the development of fatty liver disease such as nonalcoholic steatohepatitis (NASH). In this regard it is important to note that CYP2E1 is highly inducible by transcriptional, post-translational, and substrate stabilization mechanisms. Therefore, it has been hypothesized that events leading to increased expression of CYP2E1 will predispose individuals to liver disease.
Ketones and other small organic molecules are both substrates and inducers of CYP2E1 [9, 10]. It has also been hypothesized that the production of ketones by diabetics would result in increased expression and catalytic activity of CYP2E1 [11]. Numerous studies in animal models have confirmed the induction of CYP2E1 in diabetes, but this was reversed by insulin treatment. However, human data are lacking. Song and coworkers demonstrated that CYP2E1 mRNA was elevated in lymphocytes cultured from the blood of Type I diabetics [11]. However, Lucas and coworkers suggested that the chlorzoxazone metabolic ratio, a marker of CYP2E1 activity, was increased only in a subset of diabetics with a body mass index (BMI) ≥ 30 kg m−2[12]. It remains unclear whether diabetes per se induces hepatic CYP2E1 in humans. Therefore, this study was designed to investigate the expression and activity of CYP2E1 in Type I and obese Type II diabetics compared with healthy volunteers.
Materials and methods
Materials
Chlorzoxazone, 6-hydroxychlorazoxazone and phenacetin were purchased from Sigma-Aldrich Chemical Co. (St Louis, MO, USA). All enzymes and cofactors used for the synthesis of internal standard RNA by reverse transcription and PCR amplification were purchased from Promega (Madison, WI, USA), and Integrated DNA Technologies (Coralville, WI, USA) synthesized all primers. The TRI Reagent for RNA isolation was obtained from Molecular Research Center, Inc. (Cincinnati, OH, USA). All other consumables were of the highest grades available and obtained from standard commercial sources.
Study design
After approval by the institutional review board of Indiana University Purdue University at Indianapolis, 10 healthy volunteers, 14 Type I diabetics (insulin-dependent diabetes mellitus), and 8 obese Type II diabetics (noninsulin-dependent diabetes mellitus) provided written informed consent to participate in the study. Volunteers were characterized by: (i) no significant medical conditions as assessed by medical history, physical examination and blood and urine chemistry screens; (ii) no long-term use of medications; and (iii) no known allergies to chlorzoxazone. In the case of the diabetics, none had significant end-organ damage, and none was taking a known inhibitor, inducer or substrate of CYP2E1.
Demographic information for all volunteers including coadministered drugs for the Type I and obese Type II diabetics is provided in Table 1. The participants were between 18 and 65 years of age and were nonsmokers. Individuals were asked to refrain from alcohol for at least a week prior to the study. Participants were excluded for the following reasons: allergy to chlorzoxazone, a history of alcohol abuse or drug abuse. Women were excluded if they had a positive pregnancy test or if they were lactating. In addition, glycosylated haemoglobin was determined in all diabetic volunteers.
Table 1.
Clinical details and concurrent medications of the Type I and obese Type II diabetics (mean ± s.d.).
| Healthy | Type I | Obese Type II | |
|---|---|---|---|
| n | 10 | 14 | 8 |
| Sex (M/F) | 6/4 | 8/6 | 5/3 |
| Race | 6 Caucasian | 11 Caucasian | 4 Caucasian |
| 2 Hispanic, 2 Asian | 3 African-American | 4 African-American | |
| Age (years) | 32 ± 4 | 33 ± 8 | 46 ± 9* |
| Weight (kg) | 74.1 ± 10.5 | 77.2 ± 12.3 | 106.6 ± 17.6* |
| BMI | 26.6 ± 3.5 | 25.9 ± 3.9 | 37.5 ± 6.4* |
| Fasting glucose (mg dL−1) | 83 ± 10 | 173 ± 114 | 199 ± 143 |
| Cholesterol (mg dL−1) | 160 ± 31 | 182 ± 39 | 219 ± 57* |
| HbA1c††† | nd† | 9.3 ± 2.8 | 7.6 ± 3.5 |
| Concurrent medication | |||
| Drug category | Healthy | Type I | Obese Type II |
| Antidiabetic agents | |||
| Insulin | n.a.‡ | 14 | 4 |
| Other§ | n.a. | 3 | |
| Renin angiotensin antagonists¶ | n.a. | 4 | 3 |
| Antihyperlipidaemic** | n.a. | 1 | 1 |
| Diuretic†† | n.a. | 2 | |
| Hormones‡‡ | n.a. | 1 | 2 |
| Antihypertensive§§ | n.a. | 2 | |
| Antidepressant¶¶ | n.a. | 1 | 1 |
| Miscellaneous*** | n.a. | 2 | 1 |
Significantly (P≤ 0.05) different to healthy volunteers by anova with Tukey's correction.
Not determined.
Not applicable.
Metformin (2), glimepiride (2), and one obese type II diabetic volunteer was successfully controlling their diabetes with diet modification.
Drugs taken include (no. of people) benazepril, captopril, enalapril, lisinopril, ramipril, irbesartan, losartan.
Gemfibrozil, atorvastatin.
Furosemide, hydrochlorthiazide/triamterene.
Oestrogen, levothyroxine, methimazole.
Clonidine, verapamil. Nefazadone, sertraline.
Aspirin, coumarin, oxycodone and montelukast.
HbA1c (glycosylated hemoglobin) normal range is 4.8–8.0%.
Volunteers were admitted to the General Clinical Research Center at Indiana University Hospital, where they remained for the duration of the study (approximately 24 h). An intravenous catheter was placed in one forearm of each individual for the withdrawal of blood samples. Volunteers consumed a light breakfast containing no concentrated sweets. Two hours later, the participants were given a single dose of chlorzoxazone (500 mg), then serial blood samples (7 ml) were collected predose and 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, and 24 h after dosing. Urine was collected over the following intervals: predose and 0–2, 2–4, 4–6, 6–10, and 10–24 h following drug administration. Serum was harvested and stored at −20°C until analysis. Likewise, urine samples were stored at −20°C until analysis.
Analytical methods
Serum
Chlorzoxazone and 6-hydroxychlorzoxazone serum concentrations were analysed by h.p.l.c. as described previously [13]. Following the addition of internal standard, phenacetin (50 µl of a 50-µg ml−1 solution), 1 ml of a 1-m potassium phosphate:citric acid buffer pH 7, and 3 ml of ethylacetate to the serum sample, the resultant mixture was mixed vigorously for 15-30 s using a platform mixer. The sample was centrifuged at 1600 g for 10 min (Sorvall RT6000D; Kendro Laboratory Products, Newton, CT, USA), the organic layer transferred to a clean test-tube and evaporated to dryness under a vacuum (Savant Roto-vap; Thermo Savant, Holbrook, NY, USA). The residue was reconstituted with 150-300 µl of mobile phase [50 mm potassium phosphate containing 100 mm sodium acetate pH 3.5 and acetonitrile (72 : 28 v/v)] of which an aliquot was injected onto an h.p.l.c. column. Separation was accomplished with a Beckman Ultrasphere™ C-8 column (5 µm × 4.6 mm i.d. × 250 mm) equipped with a Brownlee RP-8 guard column. The mobile phase was delivered isocratically at a flow rate of 1.5 ml min−1 to a photodiode array detector (Beckman 168 photodiode array detector; Beckman Coultor, Inc., Fullerton, CA, USA) interfaced with a Beckman 125 binary pump equipped with a Beckman 508 autosampler. Chlorzoxazone, 6-hydroxychlorzoxazone, and phenacetin were monitored at 272 nm. The lower limit of quantification was 0.025 µg ml−1 for both 6-hydroxychlorzoxazone and chlorzoxazone. The interday coefficient of variation for chlorzoxazone and 6-hydroxychlorzoxazone at 0.14, 1.4, and 8.0 µg ml−1 was < 7%. Quality control samples with nominal values of 0.14, 1.4, and 8 µg ml−1 were within 10% of the expected value for both the parent drug and metabolite.
Urine
Briefly, 0.5 ml of urine was combined with 0.2 ml of 0.2 m sodium acetate pH 5.0, 10 µl of 0.6 m sodium azide, and 2000 U of β-glucuronidase and incubated at 37°C for 1 h. The samples were processed as described above following deconjugation with β-glucuronidase. The interday coefficient of variation for 6-hydroxychlorzoxazone at 1.4, 14.0, and 40.0 µg ml−1 was < 5%. Quality control samples at the same concentrations were within 8% of the expected value.
Isolation of blood mononuclear fraction
Lymphocytes were isolated from 25 ml of heparinized blood using 10 ml of Isolymph (Gallard-Schlesinger Industries, Carle Place, NY, USA) as directed by the manufacturer. Mononuclear cells containing mainly lymphocytes (> 75%) and 12-25% monocytes were counted, and the remaining portion of the pellet was dissolved in TRI Reagent and stored at −80°C until RNA isolation.
RNA isolation and cDNA synthesis
Total RNA was prepared from the peripheral mononuclear cells as described previously [14] Total RNA was isolated by a single-step procedure, and cDNA was synthesized by reverse transcriptase. The gene-specific primers were designed to amplify a specific region of CYP2E1 (Table 2), and the competitor DNA was produced by using competitive DNA primers (Table 2). Subsequently competitor DNA was transcribed to competitive reference standard RNA by T7 RNA polymerase according to the manufacturer's instructions. The concentration of competitive reference standard RNA was determined by a spectrophotometer (A260 nm). Competitive RT-PCR was performed as previously described. Briefly, in an initial experiment, logarithmic dilutions (109−103 molecules µl−1) of competitive reference standard RNA were used with a constant amount of target RNA to find a range where competitive product was formed in an approximately equal amount to that of the target mRNA. Using avian myeloid leukaemia virus reverse-transcriptase, the reverse-transcription reaction was then carried out. After terminating the reverse transcription reaction by heating to 95°C, 15 µl of PCR master mixture containing 1 mm dNTP, 10 pmol primer (Table 2), and 1 U Taq DNA polymerase were added. The reaction was terminated after 32 cycles, and PCR products were separated with a 2% agarose gel containing ethidium bromide. The gel digital image was generated using Doc 1000 u.v. Fluorescent System (BioRad Labs, Hercules, CA, USA), where molecular analysis software (BioRad) was used to quantify the band intensity. The standard curve was prepared by plotting the log ratio of target band intensity to competitive standard reference RNA against log competitive standard reference RNA, and the amount of target mRNA was determined using this standard curve. The procedure was repeated in triplicate for each sample and mean values were taken. The coefficient of variation for the quantification of CYP2E1 mRNA was 15% at 8750 CYP2E1 mRNA molecules.
Table 2.
Sequences of cytochrome P450 2E1 and Multidrug Resistance-1 mRNA forward and reverse oligonucleotide primers and sequence of crs-primers for synthesizing internal competitive standard.
| Gene | Oligonucelotide sequence (5′-3′) | Location | |
|---|---|---|---|
| CYP2E1 | Forward | AGC ACA ACT CTG AGA TAT GG | 925-944 |
| Reverse | ATA GCT ACT GTA CTT GAA CT | 1271-1290 | |
| crs-CYP2E1 | Forward | CAT TTA ATA CGA CTC ACT ATA GGG ACC TAC ATG GAT GCT GTG GTG | T7 polymerase sequence |
| Reverse | TAT AGG GCT GAG GTC GAT ATC CTT CAC ACT CGT TTT CCT GTG GA | 1432-1413 & 1409-1396 |
*NIDDM volunteer was successfully controlling their diabetes with dietary modification. †Female volunteer, African American volunteer. ‡Glycosylated haemoglobin normal range 4.8–8.0%.
Pharmacokinetic and statistical analysis
Standard model independent methods were used to determine the pharmacokinetic parameters of interest (WinNonlin, version 3.2; Pharsight, Mountain View, CA, USA). The area under the serum concentration vs time curve from zero to infinity (AUC∞0) was calculated using a combination of trapezoidal and log trapezoidal methods up to the last data point, followed by extrapolation to infinity using the terminal elimination rate constant. The latter was calculated using the slope of the terminal log-linear decline in blood concentrations with time. Terminal half-life was calculated from 0.693 divided by the terminal elimination rate constant.
Statistical analysis
Data are reported as mean ± s.d. The effects of insulin-dependent and noninsulin-dependent diabetes mellitus on the various pharmacokinetic parameters were assessed by one-way anova, and the groups responsible for an effect were identified by Tukey's multiple-comparison test (JMP® Statistics, Version 4.02; SAS Institute, Cary, NC, USA) at the significance level of 5%. For continuous variables, we used univariate linear regression analysis.
Results
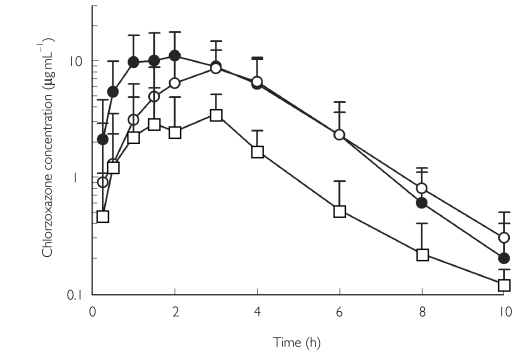
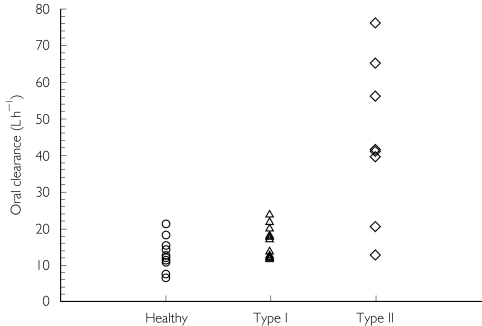
No volunteers failed to complete the study and no adverse events were observed following chlorzoxazone administration. Obese Type II diabetics were significantly older (45 ± 9 years) and heavier (Table 1) than Type I diabetics (33 ± 8 years) and healthy volunteers (32 ± 4 years). Mean plasma concentration-time profiles of chlorzoxazone are shown in Figure 1, and the corresponding pharmacokinetic parameters are presented in Table 3. The AUC(0,∞) of chlorzoxazone was reduced by 25% in Type I diabetics, and reduced significantly by 70% in obese Type II diabetics compared with healthy volunteers. Similar changes in Cmax were observed in both types of diabetes, but statistical difference was only observed in obese Type II diabetes when compared with healthy volunteers. The oral clearance of chlorzoxazone was increased significantly by 250% in obese Type II diabetics compared with healthy volunteers and 160% compared with Type I diabetics (Figure 2). After normalization for body weight, there was still a 140% increase in the oral clearance of chlorzoxazone in obese Type II diabetics compared with healthy participants. The protein binding of chlorzoxazone was not different among the three groups (Table 3). Neither the presence of Type I nor of Type II diabetes resulted in significant changes in the observed elimination half-life or the time to maximum serum concentration (Tmax) compared with healthy volunteers (Table 3). Serum 6-hydroxychlorzoxazone concentrations were not quantifiable in 9/10 healthy volunteers, but were measurable in 2–4 samples in individuals with Type 1 or Type II diabetes. The peak 6-hydroxychlorzoxazone to chlorzoxazone serum concentration ratio was significantly greater in obese Type II diabetics compared with Type I diabetics (Table 3). The percentage of the dose excreted into the urine as 6-hydroxychlorzoxazone was not significantly different between the three groups (Table 3). These observations are consistent with an increased oral clearance of chlorzoxazone rather than diminished chlorzoxazone absorption in diabetic subjects.
Figure 1.
The disposition of chlorzoxazone following an oral 500-mg dose in ten healthy volunteers, 14 volunteers with Type I diabetes mellitus and eight volunteers with Type II diabetes mellitus. Each point represents the mean (± s.d.). •, Values for healthy volunteers; ○, values for patients with Type I diabetes mellitus; □, values for patients with Type II diabetes mellitus.
Table 3.
Mean (± s.d.) pharmacokinetic parameter estimates of chlorzoxazone (500 mg) after oral administration in 32 volunteers.
| HealthyMean ± s.d. (90% CI*) | Type IMean ± s.d. (90% CI) | Obese Type IIMean ± s.d.(90% CI) | Healthyvs. Type I | 90% CI on the difference†Healthyvs. Type II | Type Ivs. Type II | |
|---|---|---|---|---|---|---|
| n | 10 | 14 | 8 | |||
| AUC∞0 (mg· h l−1) | 43.5 ± 16.9 | 32.8 ± 9.2 | 15.7 ± 11.3‡ | 1.50, 19.88 | 15.6, 39.99 | 9.45, 24.76 |
| (36.0-50.8) | (28.7-36.8) | (9.1-22.2) | ||||
| CL/F (l h−1) | 13.0 ± 4.5 | 16.7 ± 5.7 | 43.8 ± 21.4‡ | −7.47, 0.02 | −38.28, −10.89 | −32.47, −9.24 |
| (11.0-15.0) | (14.2-19.2) | (31.4-56.2) | ||||
| CL/F (l h−1 kg−1) | 0.18 ± 0.06 | 0.23 ± 0.14 | 0.43 ± 0.26‡ | −0.13, 0.02 | −0.39, −0.11 | −0.34, −0.06 |
| (0.15-0.20) | (0.17-0.29) | (0.28-0.58) | ||||
| Fraction unbound (%) | 3.9 ± 2.1 | 4.0 ± 1.3 | 3.2 ± 2.6 | −2.28, 0.67 | −2.21, 2.20 | −0.87, 2.47 |
| (2.2-4.1) | (3.4-4.5) | (1.6-4.7) | ||||
| Half-life (h) | 1.1 ± 0.2 | 1.2 ± 0.4 | 1.4 ± 0.9 | −0.38, 0.13 | −0.79, 0.24 | −0.64, 0.34 |
| (1.0-1.2) | (1.1-1.5) | (0.9-2.0) | ||||
| Tmax (h) | 2.1 ± 1.0 | 2.7 ± 1.0 | 2.3 ± 1.1 | −1.26, 0.10 | −1.05, 0.63 | −0.39, 1.13 |
| (1.7-2.5) | (2.3-3.1) | (1.7-2.9) | ||||
| Cmax (µg ml−1) | 15.0 ± 3.7 | 12.2 ± 4.4 | 5.2 ± 2.2‡ | −0.33, 5.81 | 7.21, 12.35 | 4.01, 10.07 |
| (13.4-16.6) | (10.2-14.1) | (3.9-6.5) | ||||
| Vd/F (l kg−1) | 0.28 ± 0.08 | 0.40 ± 0.16 | 0.70 ± 0.26‡ | −0.21, 0.02 | −0.57, −0.27 | −0.45, −0.17 |
| (0.25-0.32) | (0.33-0.47) | (0.55-0.85) | ||||
| Peak 6-OHCHZ/CHZ serum conc. ratio | 0.029§ | 0.010 ± 0.007 | 0.028 ± 0.012¶ | n.a. | n.a. | −0.03, −0.01 |
| (0.007-0.013) | (0.022-0.033) | |||||
| Urinary recovery (% of dose) | 69.7 ± 7.9 | 61.4 ± 12.0 | 64.8 ± 13.5 | −0.83, 15.83 | −4.44, 14.21 | −13.98, 7.09 |
| (66.2-73.2) | (56.1-66.7) | (58.9-70.7) |
CI, Confidence interval; 6-OHCHZ, 6-hydroxychlorzoxazone; CHZ, chlorzoxazone; conc., concentration; n.a., not applicable.
The 90% confidence intervals are based on t-test with equal variance.
Values significantly different (P≤0.05) from healthy and Type I values.
Value for one volunteer only, 6-hydroxychlorzoxazone not detectable in 9/10 healthy individuals.
Value significantly different from Type I value.
Figure 2.
The effect of Type I and Type II diabetes on chlorzoxazone oral clearance, a marker of CYP2E1 activity in healthy volunteers (n = 10), Type I diabetics (n = 14) and Type II diabetics (n = 8).
The mRNA of CYP2E1 was significantly (P ≤ 0.05) elevated in the lymphocytes from patients with Type 1 (9587 ± 6729 copies µg−1 mRNA) and obese Type II diabetes (15997 ± 2321 copies µg−1 mRNA) compared with healthy volunteers (1962 ± 1942). However, there was no relationship between lymphocyte CYP2E1 mRNA content and oral chlorzoxazone clearance (data not shown).
We examined the effect of the covariates weight, height, BMI, serum glucose, serum cholesterol, and glycosylated haemoglobin on the estimated pharmacokinetic parameters of chlorzoxazone. The oral clearance of chlorzoxazone was not significantly influenced by bodyweight, BMI, or any of the other covariates tested. Likewise, there was no relationship between oral chlorzoxazone clearance (l h−1 or l h−1 kg−1) and baseline blood glucose concentrations or glycosylated haemoglobin A1c in diabetic individuals (data not shown). The AUC∞0 demonstrated a significant inverse relationship with serum glucose concentration in all individuals (r = 0.41, n = 32, P ≤ 0.05). In obese Type II diabetics increasing BMI was associated with an increasing Cmax. However, in Type I diabetics and healthy controls the opposite relationship was observed. No significant relationships were observed between Tmax and half-life and BMI, weight, height, serum cholesterol, serum glucose concentration, or glycosylated haemoglobin.
Discussion
Chlorzoxazone is a potent skeletal muscle relaxant that is effective in the treatment of skeletal muscle spasms. Onset of therapeutic activity is observed within 1 h, with a duration of action of approximately 6 h [15]. CYP2E1 is the principal catalyst of the 6-hydroxylation of chlorzoxazone and therefore has been advocated as an in vivo CYP2E1 probe [5]. Although other enzymes such as CYP1A2 and CYP3A have been implicated in the in vitro metabolism of chlorzoxazone, these observations have not been confirmed in vivo[13, 16]. For example, the coadministration of caffeine and chlorzoxazone resulted in a 16% change in the caffeine to paraxanthine metabolic ratio (an index of CYP1A2 activity), but no change in chlorzoxazone kinetics [17]. Likewise, chlorzoxazone has been shown to minimally influence the disposition of midazolam (a CYP3A substrate) in vivo, although this is controversial [18, 19].
CYP2E1 may be responsible, in part, for the liver toxicity associated with paracetamol and ethanol intake by forming reactive metabolites such as NAPQI [20–22]. CYP2E1 has been identified as a source of reactive oxygen species, which are thought to cause lipid peroxidation resulting in liver damage such as that found in NASH and alcoholic liver disease. Although a causal relationship between CYP2E1 and NASH has not been confirmed, it has been shown that CYP2E1 protein concentrations and the rates of lipid peroxidation are elevated in these individuals [7, 8, 23]. Finally, individuals with NASH appear to be more susceptible to paracetamol hepatotoxicity, presumably due to the up-regulation of CYP2E1.
Clinically, diabetes mellitus is categorized into two types, one arising from an insulin deficiency (Type I, insulin-dependent diabetes mellitus), the other from insulin resistance (Type II, noninsulin-dependent diabetes mellitus). In animals, hepatic CYP2E1 expression and activity can be modulated by genetically determined obesity, diet (high fat intake, fasting), or by the administration of xenobiotics (isoniazid, ethanol) [24–28]. It is clear that chemically (streptozotocin) induced diabetes mellitus consistently results in an increased expression of hepatic and lymphocytic CYP2E1 protein and mRNA, and alterations in other drug-metabolizing enzymes in rodents, which are partly a result of increased concentrations of circulating ketones [9, 10, 26, 29, 30]. The latter have been shown to be an important modulator of CYP2E1, causing enhanced expression through mRNA or protein stabilization [31]. These changes in the drug-metabolizing enzymes of rodents are reversed by the administration of insulin [32]. However, it is unclear if these animal models of diabetes accurately reflect the molecular mechanisms responsible for the development of diabetes in humans.
In the present study, we did not observe a significant difference in the oral clearance of chlorzoxazone between healthy volunteers and Type I diabetics. This is somewhat unexpected given the changes in circulating ketones and CYP2E1 expression and activity observed in animal models of Type I (chemically induced) diabetes. However, in these models of Type I diabetes the animals do not receive insulin, which has been shown to correct the changes in circulating ketones and return CYP2E1 expression to basal levels. In this study, the Type I diabetics received insulin supplementation as part of their pharmacotherapy, and no one had substantial ketonuria prior to the study, although three of 14 individuals had trace amounts of ketones in their urine. Thus, our data indicate that Type I diabetics under moderate control (glycosylated haemoglobin A1c= 8.4 ± 2.9) have similar chlorzoxazone oral clearances compared with healthy controls. No relationship was observed between glycosylated haemoglobin and the oral clearance of chlorzoxazone. However, we observed an inverse relationship between chlorzoxazone area under the curve and fasting blood glucose concentrations. The role of the latter in CYP2E1 expression and activity has not been defined. Our observation is consistent with the finding of Leclercq and coworkers, who reported that dietary sugar restriction decreased CYP2E1 activity, as assessed by chlorzoxazone exposure [33]. Additionally, this observation may indirectly reflect the role of insulin in the regulation of CYP2E1. Woodcroft and colleagues demonstrated that expression and activity of CYP2E1 in cultured rat hepatocytes exhibited a dose-response relationship with insulin [34].
It is well known that there is a relationship between obesity and the incidence of Type II diabetes. O'Shea et al. observed that obese individuals had a 50% increase in the oral clearance of chlorzoxazone compared with nonobese individuals [35]. Likewise, Lucas and colleagues demonstrated a significant 40% increase in the 2-h 6-hydroxychlorzoxazone to chlorzoxazone serum concentration ratio in obese Type II diabetics compared with healthy volunteers and Type I diabetics [12]. Furthermore, this CYP2E1 metabolic index demonstrated a positive relationship with BMI, serum triglycerides and serum cholesterol in all subjects [12]. In agreement with these observations we observed that the oral chlorzoxazone clearance was increased by 3.4-fold in Type II diabetics compared with healthy volunteers. In contrast to the findings of Lucas and colleagues, we did not observe significant relationships between the oral clearance of chlorzoxazone and BMI, serum cholesterol, or other covariates in the three groups.
Although there was a parallel decrease in the area under the plasma concentration-time curve and Cmax of chlorzoxazone in both types of diabetes mellitus, especially obese Type II diabetics, no change in the elimination half-life (Table 3) was observed between the three groups. The lack of change in the elimination half-life may reflect an increased first-pass metabolism or concomitant increases in the volume of distribution and systemic clearance of chlorzoxazone. Assuming a well-stirred model of elimination with the liver as the sole organ of systemic elimination then:
The oral clearance (CLPO) is equal to the product of the fraction unbound (fu) and intrinsic clearance (CLint). Oral availability (FPO) is the product of three determinants, hepatic availability (FH), intestinal availability (FI) and the fraction of the dose absorbed (FABS). A reduction in the oral availability mediated by a decrease in the fraction of the dose absorbed, or increases in the intrinsic clearance and fraction unbound, will result in an increase in the oral clearance of a drug. In this study, the fraction of the dose absorbed (FABS) does not appear to be reduced in obese Type II diabetics, because the fraction of the dose excreted into the urine was not different between healthy and diabetic volunteers. Although the intestine is capable of contributing to the first-pass metabolism of a number of CYP3A substrates, such as midazolam, it does not express CYP2E1 protein. Thus, FI is unlikely to be altered (personal communication, K. Thummel, University of Washington) [36]. Additionally, the contribution of CYP3A to chlorzoxazone biotransformation is small and neither diabetes nor obesity have been linked to increased CYP3A expression. The fractions unbound in healthy Type I diabetic, and obese Type II diabetic volunteers were equivalent. Finally, the lack of alteration in protein binding in obese Type II diabetics suggests that increases in both the systemic clearance and the volume of distribution of equivalent magnitude are unlikely. Likewise, the greater peak 6-hydroxychlorzoxazone to chlorzoxazone serum concentration ratios observed in obese Type II diabetics compared with Type I diabetics suggests that CYP2E1 expression and activity are increased. Thus, the study findings indicate that changes in the oral clearance of chlorzoxazone probably reflect enhanced hepatic CYP2E1 expression and activity.
In addition, we observed an increase in CYP2E1 mRNA expression in peripheral mononuclear cells in both types of diabetes mellitus. These results are consistent with the observation that diabetes mellitus itself can lead to changes in CYP2E1 expression and activity. Although, we observed changes in CYP2E1 mRNA expression in peripheral mononuclear cells, this may not be indicative of hepatic changes. Inconsistent with a previous report, CYP2E1 mRNA in the peripheral mononuclear cells is elevated as a result of diabetes mellitus, but there is no correlation between the apparent oral clearance of chlorzoxazone and CYP2E1 mRNA content [37]. This may be due to the different regulatory mechanism of CYP2E1 gene expression in the mononuclear cells and hepatocytes, and additional translation and post-translational regulation of CYP2E1 expression may occur.
In conclusion, this study demonstrates that obese Type II diabetics have enhanced CYP2E1 catalytic activity compared with healthy volunteers and Type I diabetics as assessed by chlorzoxazone oral clearance. In contrast, Type I diabetics under moderate control exhibited CYP2E1 activity comparable to healthy volunteers. We observed no relationship between chlorzoxazone oral clearance and BMI and serum cholesterol, but noted an inverse relationship between serum glucose and CYP2E1 activity. The enhanced CYP2E1 activity observed in Type II diabetics may contribute to the adverse hepatic events associated with this disease.
Acknowledgments
Supported by NIH Grants T32GM08425, M01-RR00750, and the FDA Co-operative agreement FDT-001756.
References
- 1.Nelson DR, Koymans L, Kamataki T, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996;6:1–42. doi: 10.1097/00008571-199602000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Wrighton SA, Stevens JC. The human hepatic cytochromes P450 involved in drug metabolism. Crit Rev Toxicol. 1992;22:1–21. doi: 10.3109/10408449209145319. [DOI] [PubMed] [Google Scholar]
- 3.Guengerich FP, Kim DH, Iwasaki M. Role of human cytochrome P-450 IIE1 in the oxidation of many low molecular weight cancer suspects. Chem Res Toxicol. 1991;4:168–179. doi: 10.1021/tx00020a008. [DOI] [PubMed] [Google Scholar]
- 4.Surbrook SE, Jr, Olson MJ. Dominant role of cytochrome P-450 2E1 in human hepatic microsomal oxidation of the CFC-substitute 1,1,1,2-tetrafluoroethane. Drug Metab Dispos. 1992;20:518–524. [PubMed] [Google Scholar]
- 5.Peter R, Bocker R, Beaune PH, et al. Hydroxylation of chlorzoxazone as a specific probe for human liver cytochrome P-450IIE1. Chem Res Toxicol. 1990;3:566–573. doi: 10.1021/tx00018a012. [DOI] [PubMed] [Google Scholar]
- 6.Kharasch ED, Thummel KE. Identification of cytochrome P450 2E1 as the predominant enzyme catalyzing human liver microsomal defluorination of sevoflurane, isoflurane, and methoxyflurane. Anesthesiology. 1993;79:795–807. doi: 10.1097/00000542-199310000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 8.Niemela O, Parkkila S, Juvonen RO, et al. Cytochromes P450 2A6, 2E1, and 3A and production of protein-aldehyde adducts in the liver of patients with alcoholic and non-alcoholic liver diseases. J Hepatol. 2000;33:893–901. doi: 10.1016/s0168-8278(00)80120-8. [DOI] [PubMed] [Google Scholar]
- 9.Barnett CR, Petrides L, Wilson J, Flatt PR, Ioannides C. Induction of rat hepatic mixed-function oxidases by acetone and other physiological ketones: their role in diabetes-induced changes in cytochrome P450 proteins. Xenobiotica. 1992;22:1441–1450. doi: 10.3109/00498259209056694. [DOI] [PubMed] [Google Scholar]
- 10.Shimojo N, Ishizaki T, Imaoka S, et al. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochem Pharmacol. 1993;46:621–627. doi: 10.1016/0006-2952(93)90547-a. [DOI] [PubMed] [Google Scholar]
- 11.Song BJ, Veech RL, Saenger P. Cytochrome P450IIE1 is elevated in lymphocytes from poorly controlled insulin-dependent diabetics. J Clin Endocrinol Metab. 1990;71:1036–1040. doi: 10.1210/jcem-71-4-1036. [DOI] [PubMed] [Google Scholar]
- 12.Lucas D, Farez C, Bardou LG, et al. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam Clin Pharmacol. 1998;12:553–558. doi: 10.1111/j.1472-8206.1998.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 13.Gorski JC, Jones DR, Wrighton SA, Hall SD. Contribution of human CYP3A subfamily members to the 6-hydroxylation of chlorzoxazone. Xenobiotica. 1997;27:243–256. doi: 10.1080/004982597240578. [DOI] [PubMed] [Google Scholar]
- 14.Asghar A, Haehner-Daniels BD, Gorski JC, Hall SD. Induction of MDR1 and cytochrome P450 mRNAs by rifampin in human mononuclear cells. Drug Metab Dispos. 2002;30:20–26. doi: 10.1124/dmd.30.1.20. [DOI] [PubMed] [Google Scholar]
- 15.Desiraju RK, Renzi NL, Jr, Nayak RK, Ng KT. Pharmacokinetics of chlorzoxazone in humans. J Pharm Sci. 1983;72:991–994. doi: 10.1002/jps.2600720905. [DOI] [PubMed] [Google Scholar]
- 16.Ono S, Hatanaka T, Hotta H, et al. Chlorzoxazone is metabolized by human CYP1A2 as well as by human CYP2E1. Pharmacogenetics. 1995;5:143–150. doi: 10.1097/00008571-199506000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Berthou F, Goasduff T, Lucas D, et al. Interaction between two probes used for phenotyping cytochromes P4501A2 (caffeine) and P4502E1 (chlorzoxazone) in humans. Pharmacogenetics. 1995;5:72–79. doi: 10.1097/00008571-199504000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Palmer JL, Scott RJ, Gibson A, Dickins M, Pleasance S. An interaction between the cytochrome P450 probe substrates chlorzoxazone (CYP2E1) and midazolam (CYP3A) Br J Clin Pharmacol. 2001;52:555–561. doi: 10.1046/j.0306-5251.2001.01479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu B, Ou-Yang DS, Chen XP, et al. Assessment of cytochrome P450 activity by a five-drug cocktail approach. Clin Pharmacol Ther. 2001;70:455–461. doi: 10.1067/mcp.2001.119813. [DOI] [PubMed] [Google Scholar]
- 20.Thummel KE, Lee CA, Kunze KL, Nelson SD, Slattery JT. Oxidation of acetaminophen to N-acetyl-p-aminobenzoquinone imine by human CYP3A4. Biochem Pharmacol. 1993;45:1563–1569. doi: 10.1016/0006-2952(93)90295-8. [DOI] [PubMed] [Google Scholar]
- 21.Dupont I, Lucas D, Clot P, Menez C, Albano E. Cytochrome P4502E1 inducibility and hydroxyethyl radical formation among alcoholics. J Hepatol. 1998;28:564–571. doi: 10.1016/s0168-8278(98)80279-1. [DOI] [PubMed] [Google Scholar]
- 22.Koop DR, Coon MJ. Ethanol oxidation and toxicity: role of alcohol P-450 oxygenase. Alcohol Clin Exp Res. 1986;10(6 Suppl):44S–49S. doi: 10.1111/j.1530-0277.1986.tb05179.x. [DOI] [PubMed] [Google Scholar]
- 23.Leclercq IA, Farrell GC, Field J, et al. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Raucy JL, Lasker JM, Kraner JC, et al. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–280. [PubMed] [Google Scholar]
- 25.Brouwer KL, Kostenbauder HB, McNamara PJ, Blouin RA. Phenobarbital in the genetically obese Zucker rat. II. In vivo and in vitro assessments of microsomal enzyme induction. J Pharmacol Exp Ther. 1984;231:654–659. [PubMed] [Google Scholar]
- 26.Enriquez A, Leclercq I, Farrell GC, Robertson G. Altered expression of hepatic CYP2E1 and CYP4A in obese, diabetic ob/ob mice, and fa/fa Zucker rats. Biochem Biophys Res Commun. 1999;255:300–306. doi: 10.1006/bbrc.1999.0202. [DOI] [PubMed] [Google Scholar]
- 27.Weltman MD, Farrell GC, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–1653. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- 28.Leclercq I, Horsmans Y, Desager JP, Delzenne N, Geubel AP. Reduction in hepatic cytochrome P-450 is correlated to the degree of liver fat content in animal models of steatosis in the absence of inflammation. J Hepatol. 1998;28:410–416. doi: 10.1016/s0168-8278(98)80314-0. [DOI] [PubMed] [Google Scholar]
- 29.Barnett CR, Gibson GG, Wolf CR, Flatt PR, Ioannides C. Induction of cytochrome P450III and P450IV family proteins in streptozotocin-induced diabetes. Biochem J. 1990;268:765–769. doi: 10.1042/bj2680765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimojo N. Cytochrome P450 changes in rats with streptozocin-induced diabetes. Int J Biochem. 1994;26:1261–1268. doi: 10.1016/0020-711x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 31.Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- 32.Favreau LV, Malchoff DM, Mole JE, Schenkman JB. Responses to insulin by two forms of rat hepatic microsomal cytochrome P-450 that undergo major (RLM6) and minor (RLM5b) elevations in diabetes. J Biol Chem. 1987;262:14319–14326. [PubMed] [Google Scholar]
- 33.Leclercq I, Horsmans Y, Desager JP, Pauwels S, Geubel AP. Dietary restriction of energy and sugar results in a reduction in human cytochrome P450 2E1 activity. Br J Nutr. 1999;82:257–262. [PubMed] [Google Scholar]
- 34.Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology. 2002;35:263–273. doi: 10.1053/jhep.2002.30691. [DOI] [PubMed] [Google Scholar]
- 35.O'Shea D, Davis SN, Kim RB, Wilkinson GR. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: a putative probe of CYP2E1 activity. Clin Pharmacol Ther. 1994;56:359–367. doi: 10.1038/clpt.1994.150. [DOI] [PubMed] [Google Scholar]
- 36.Zhang QY, Dunbar D, Ostrowska A, et al. Characterization of human small intestinal cytochromes P-450. Drug Metab Dispos. 1999;27:804–809. [PubMed] [Google Scholar]
- 37.Raucy JL, Schultz ED, Wester MR, et al. Human lymphocyte cytochrome P450 2E1, a putative marker for alcohol-mediated changes in hepatic chlorzoxazone activity. Drug Metab Dispos. 1997;25:1429–1435. [PubMed] [Google Scholar]