Abstract
Aims
The objectives of this study were: (i) to evaluate the effect of a cytochrome P450 (CYP) 3A4 inhibitor, erythromycin, on the pharmacokinetics of intravenous lignocaine and its two pharmacologically active metabolites, monoethylglycinexylidide (MEGX) and glycinexylidide (GX); (ii) to assess whether the effects of the erythromycin inhibitory action on lignocaine clearance and the results of the MEGX liver function test depend on liver functional status; and (iii) to determine the effects of both moderate and severe liver dysfunction on the disposition kinetics of lignocaine.
Methods
The study was carried out on 10 healthy volunteers, and 10 Child's class A and 10 class C cirrhotic patients, according to a double-blind, randomized, two-way crossover design. On day 1 of the investigation, all subjects received three oral doses of erythromycin (600 mg of the ethylsuccinate ester) or placebo, and two further doses on day 2. One hour after the fourth dose, subjects were given 1 mg kg−1 lignocaine intravenously. Timed plasma samples were then obtained until 12 h for determination of the concentrations of lignocaine, MEGX and GX.
Results
Erythromycin caused statistically significant, although limited, modifications of lignocaine and MEGX pharmacokinetic parameters. In healthy volunteers, lignocaine clearance was decreased from 9.93 to 8.15 ml kg−1 min−1[mean percentage ratio (95% CI), 82 (65–98)] and the half-life was prolonged from 2.23 to 02.80 h [mean percentage ratio (95% CI), 130 (109–151)]; MEGX area under the concentration-time curve from 0 h to 12 h was increased from 665 to 886 ng ml−1 h [mean percentage ratio (95% CI), 129 (102–156)]. Quantitatively similar modifications were observed in the two cirrhotic groups. GX concentrations were lowered in all study groups, although not to statistically significant extents. Erythromycin coadministration caused no appreciable interference with the results of the MEGX test. Only in patients with Child's grade C liver cirrhosis were lignocaine kinetic parameters significantly altered with respect to healthy volunteers. Thus, clearance was approximately halved, steady-state volume of distribution was increased, and terminal half-life was more than doubled.
Conclusions
Although erythromycin only modestly decreases lignocaine clearance, it causes a concomitant elevation of the concentrations of its pharmacologically active metabolite MEGX. A pharmacodynamic study following lignocaine infusion to steady state appears necessary to assess the actual clinical relevance of these combined effects. The degree of liver dysfunction has no influence on the extent of the erythromycin-lignocaine interaction, whereas it markedly influences the extent of the changes in lignocaine pharmacokinetics. These findings indicate that no dose adjustment is needed in patients with moderate liver cirrhosis, whereas the lignocaine dose should be halved in patients with severe cirrhosis.
Keywords: erythromycin, lignocaine, liver disease, MEGX test, pharmacokinetics
Introduction
Lignocaine is a local anaesthetic agent that is also useful in the acute intravenous treatment of ventricular arrhythmias. Owing to its high lipophilicity, it is eliminated mainly by metabolism, < 5% being excreted unchanged in urine (see [1] for a review). The principal metabolic pathway of lignocaine in human beings is oxidative de-ethylation to monoethylglycinexylidide (MEGX), which is further de-ethylated to glycinexylidide (GX). The latter is hydrolysed to xylidine and then oxidized to 4-hydroxy-xylidine, the main metabolic product found in urine [1].
Cytochrome P450 (CYP) 3A4 has been proposed as the main CYP isoform responsible for MEGX formation [2]. Erythromycin has been shown to produce quasi-irreversible inhibition of CYP3A4 in vitro, via formation of a CYP3A4-iron-metabolite complex, and to cause clinically important drug interactions with CYP3A4 substrates (see [3, 4] for recent reviews). According to a previous study in healthy volunteers [5], erythromycin causes a statistically significant but limited increase in lignocaine half-life, and a more pronounced increase in the MEGX area under the concentration-time curve. While the present study was in progress, a further investigation on healthy volunteers was published, which found no significant effect of erythromycin on lignocaine disposition kinetics [6].
The present study had the following objectives:
To evaluate the effect of erythromycin, as a prototypical CYP3A4 inhibitor, on the disposition kinetics of lignocaine in healthy volunteers and patients with liver cirrhosis. This disease causes progressive capillarization of liver sinusoids, with a drastic reduction of effective blood flow. Consequently, the flow-dependent clearance of drugs such as lignocaine becomes more and more capacity-limited [7] and, according to theoretical predictions [8], much more sensitive to the action of metabolic inhibitors. Therefore, the results obtained in healthy subjects cannot a priori be extended to patients with liver dysfunction.
To assess the possible interference of erythromycin with MEGX liver function test (involving measurement of a single MEGX concentration after intravenous (i.v.) bolus injection of lignocaine) [9].
To determine the effect of erythromycin on GX concentration, which was not measured in the two previous studies [5, 6]. These data are important for the correct interpretation of results, as the concentration of MEGX depends on both the rate of its generation from lignocaine and that of its conversion to GX. Measurement of MEGX and GX concentrations is also clinically relevant, as these metabolites retain some antiarrhythmic activity and also contribute to lignocaine toxicity [1].
To reassess the effects of both moderate and severe liver dysfunction on lignocaine pharmacokinetics. Although numerous studies have investigated the pharmacokinetics of i.v. lignocaine in patients with liver cirrhosis, only preliminary data are available on the relation of the severity of liver dysfunction to the extent of changes in lignocaine pharmacokinetics, as small numbers of patients (5–6 per cirrhotic group) have been examined [10].
To this end, the pharmacokinetics of lignocaine, MEGX and GX were studied in healthy volunteers and cirrhotic patients, stratified according to the degree of liver dysfunction, after coadministration of either placebo or therapeutic doses of erythromycin.
Methods
Subjects
Thirty male subjects (ten healthy volunteers and 20 patients with biopsy-proven liver cirrhosis) participated in the study, after giving their informed written consent. The study design was approved by the Ethics Committee of the University Hospital of Padova. Healthy volunteers were recruited from outpatients attending the hospital for routine laboratory tests. They were diagnosed as healthy by means of a thorough clinical examination, including medical history, physical examination, electrocardiogram and standard laboratory tests. All participating patients had posthepatitic cirrhosis and were clinically stable, i.e. they showed no significant changes in clinical profiles or biochemical variables during a 1-month period prior to the initiation of the study. Ten patients were in a compensated state and were categorized as Child's class A, and 10 were in a decompensated state and were categorized as Child's class C [11]. The functional reserve of the liver was scored according to Pugh et al.[12]. Patients were excluded from this study if they had a history of gastrointestinal bleeding, severe encephalopathy or any other disease. None of the participants was a smoker or a heavy consumer of alcohol. They were requested to abstain from alcohol during the preceding week and throughout the period of investigation. For ethical reasons, treatment of patients with decompensated cirrhosis with furosemide and/or canrenone was not suspended. However, none of the participants took drugs known to induce or inhibit CYP3A4 [13] or suspected to interfere with lignocaine metabolism.
Study design and procedures
The study was conducted according to a double-blind, randomized, two-way crossover design with a washout period of at least 7 days. On the morning of study day 1, after an overnight fast, all participants entered the Gastroenterology Unit of the University Hospital of Padova and received either 600 mg erythromycin ethylsuccinate or a matched placebo orally at 07.00, 15.00 and 22.00 h. On day 2 they received a fourth dose of erythromycin or placebo at 07.00 h, and 1 h later, 1 mg kg−1 intravenous lignocaine, infused over 1 min by means of a precise volumetric infusion pump. A final erythromycin or placebo dose was given at 15.00 h. A similar erythromycin dosage has been shown to decrease markedly the elimination of the CYP3A4 substrate triazolam [14].
After lignocaine administration, all subjects remained supine for 2 h. They were asked to report any subjective adverse effects and their vital signs were closely monitored. A meal low in protein and fat content (to prevent food-induced changes in liver blood flow) was provided after 4 h. Blood samples were collected through an indwelling catheter in heparinized plastic tubes at 0 (predose), 2, 5, 10, 15, 20, 30, 45 min and 1, 1.5, 2, 2.5, 3, 4, 6, 8, 10, 12 h after the end of the 1-min lignocaine infusion. Blood was centrifuged immediately after collection and plasma samples stored at −40°C until assayed. Lignocaine, MEGX and GX concentrations were determined by h.p.l.c., with ultraviolet detection as described by O'Neal and Poklis [15]. The limits of quantification were 6 ng ml−1 for MEGX and GX, and 20 ng ml−1 for lignocaine. The intra- and inter-assay coefficients of variations (CV) for MEGX and GX (n = 10) were < 10% at 10 ng ml−1 and < 5% at 100 ng ml−1. Intra- and inter-assay CVs for lignocaine were < 11% at 100 ng ml−1 and < 7% at 1000 ng ml−1. Preliminary experiments showed that erythromycin did not interfere with the assays.
Pharmacokinetic analysis
The lignocaine plasma concentration-vs-time data were modelled by means of GraphPad Prism 3.0 software. With few exceptions, the best fit was obtained using a triexponential equation. Pharmacokinetic parameters, defined in Table 2, were calculated from the coefficients and exponents of the best-fitting equations using standard formulae [16]. The pharmacokinetics of MEGX and GX were characterized by areas under the plasma concentration-time curves from 0 h to 12 h (AUC0−12), peak plasma concentrations (Cmax) and peak times (tmax). Because of the irregular shapes of the curves, AUC0−12 was calculated by means of the trapezoidal rule. Cmax and tmax were estimated from the experimental data.
Table 2.
Effect of erythromycin coadministration on the pharmacokinetic parameters of lignocaine.
| Patients with cirrhosis | ||||||
|---|---|---|---|---|---|---|
| Healthy subjects | Child's class A | Child's class C | ||||
| Parameters | Placebo | Erythromycin | Placebo | Erythromycin | Placebo | Erythromycin |
| CL (ml kg, 1 min, 1) | 9.83 ± 3.02 | 8.15 ± 3.39* | 9.56 ± 2.03 | 7.79 ± 2.43* | 5.46 ± 1.83†† | 4.26 ± 1.77**†† |
| Ratio % (95% CI) | 82 (65, 98) | 81 (63, 99) | 77 (62, 91) | |||
| Vc (l kg, 1) | 0.66 ± 0.35 | 0.61 ± 0.31 | 0.38 ± 0.18 | 0.40 ± 0.13 | 0.38 ± 0.13 | 0.42 ± 0.13 |
| Ratio % (95% CI) | 102 (79, 125) | 105 (75, 135) | 110 (89, 129) | |||
| Vss (l kg, 1) | 1.47 ± 0.27 | 1.34 ± 0.34 | 1.95 ± 0.38† | 2.08 ± 0.74† | 2.44 ± 0.72†† | 2.42 ± 0.68†† |
| Ratio % (95% CI) | 92 (77, 106) | 105 (87, 124) | 100 (89, 111) | |||
| t½ (h) | 2.23 ± 0.55 | 2.80 ± 0.45** | 3.15 ± 0.94 | 4.09 ± 2.13** | 5.77 ± 1.19††† | 7.74 ± 2.31**††† |
| Ratio % (95% CI) | 130 (109, 151) | 124 (104, 145) | 132 (118, 147) | |||
Data are presented as means ± s.d. CL, Systemic clearance; Vc, apparent volume of the central compartment; Vss, apparent volume of distribution at steady state; t1/2, elimination half-life.
P < 0.05
P < 0.01 vs. placebo.
P < 0.05
P < 0.01
P < 0.001 vs. healthy volunteers.
Statistical analysis
We first performed power analyses for both inter- and intra-group comparisons using the CSS power assessment procedure (CSS; Statsoft Inc., Tulsa, OK, USA; 1991). On the basis of the coefficients of variation obtained by Villeneuve et al.[10] for lignocaine clearance, these analyses indicated that: (i) 10 subjects should be sufficient to detect intergroup differences of 20%, with a significance level (α) of 0.05 and a power (1-β) of 0.95, and (ii) the same number of subjects should be sufficient to detect intragroup differences in clearance of 20%, with a significance level of 0.5 and a power of 0.92. Pharmacokinetic parameters and MEGX concentration values from 15–60 min (MEGX test) were then tested for normal distribution using the Wilk-Shapiro test (SAS univariate procedure; SAS release 6.03., SAS Institute, Cary, NC, USA; 1988) and for homogeneity of variances using the Levene test (CSS Levene test of homogeneity of variances). As normal distribution of the data could not be rejected, inter- and intra-group comparisons were made by one- and two-way analyses of variance (anova), respectively, using a general linear model (SAS GLM procedure). In case of significant differences (α = 0.05), the anova was followed by the Newman-Keuls multicomparison test for pairwise comparisons. For tmax, the nonparametric Kruscal-Wallis test was used. A P-value of < 0.05 was considered statistically significant.
Results
The characteristics of the subjects studied are listed in Table 1. There were no statistically significant differences between the three groups for age, weight, height, or body mass index. On the basis of the conventional liver function tests, minor differences were apparent between patients with Child's grade A cirrhosis and healthy volunteers. In contrast, test results were indicative of advanced hepatocellular insufficiency in Child's class C cirrhotic patients. No significant changes in blood pressure or pulse rate were observed after lignocaine injection. Subjective adverse effects (paraesthesia, dizziness or drowsiness) were mild and transient, and only occurred in a few subjects (1–3) in each of the three study groups.
Table 1.
Summary of patient characteristics
| Patients with cirrhosis | |||
|---|---|---|---|
| Characteristic (normal range) | Healthy subjects | Child's class A | Child's class C |
| Age (years) | 53 ± 12 | 55 ± 7 | 54 ± 9 |
| Weight (kg) | 78 ± 8 | 82 ± 10 | 79 ± 8 |
| Height (cm) | 175 ± 6 | 172 ± 6 | 170 ± 7 |
| Body mass index (kg m−2) | 25.4 ± 3.4 | 27.0 ± 2.3 | 28.0 ± 2.7 |
| Albumin (35–55 g l−1) | 41.5 ± 4.3 | 39.7 ± 3.6 | 27.9 ± 3.3** |
| Bilirubin (5–17 µmol l−1) | 13.3 ± 3.1 | 17.6 ± 5.2 | 49.9 ± 26.0** |
| Prothrombin level (70–100%) | 89.1 ± 8.4 | 81.4 ± 7.7 | 68.8 ± 14.9* |
| Pugh score (5)† | 5 (5) | 5 (5–6) | 10.5 (10–14)** |
Data are presented as means ± s.d.
P < 0.01
P < 0.001 vs. healthy subjects and Child's class A cirrhotic patients.
Median (range).
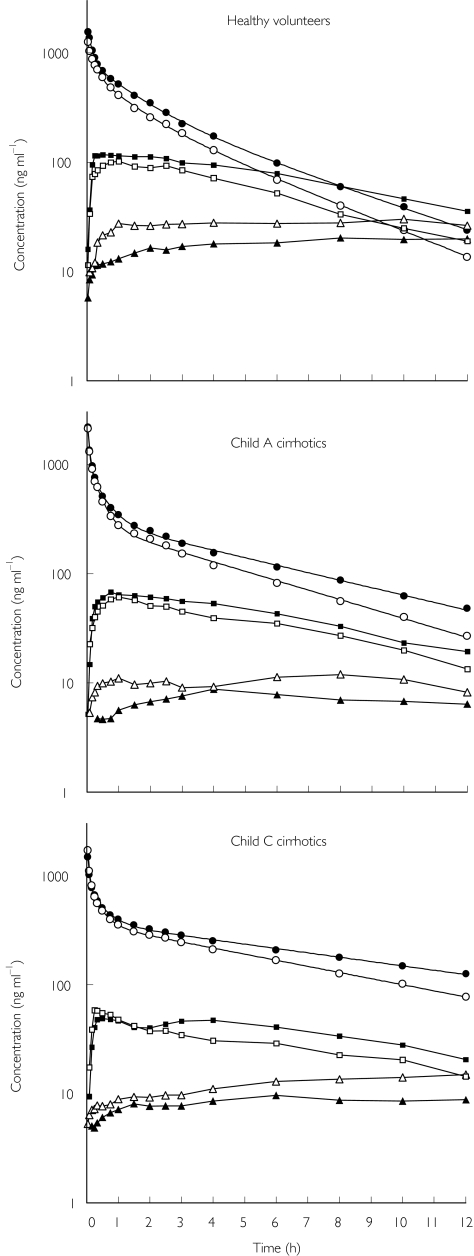
The plasma concentration vs. time profiles of lignocaine, MEGX and GX in the three study groups are shown in Figure 1. It is evident that erythromycin produced similar effects in healthy volunteers and the two groups of cirrhotic patients. Thus, it impaired lignocaine elimination, increased MEGX concentrations, and reduced those of GX. Table 2 shows that erythromycin coadministration caused a statistically significant, although modest (about 20%), reduction in lignocaine clearance and a somewhat more pronounced increase in terminal half-life, both effects being quantitatively similar in the three study groups. No significant modification of either Vss or Vc was observed. Table 2 also indicates that only in decompensated (Child's class C) cirrhotic patients were the disposition kinetics of lignocaine profoundly altered compared with healthy volunteers. In particular, CL was approximately halved, whereas Vss was increased and, consequently, t1/2 was more than doubled. As shown in Table 3, MEGX AUC0−12 increased significantly (about 30%) following erythromycin administration, whereas GX AUC0−12 decreased, but to a lesser and statistically nonsignificant extent. The effect of liver dysfunction was manifest in both Child's class A and C cirrhotic patients, with significant reductions in both MEGX and GX AUC0−12. A marked prolongation of MEGX tmax was also observed in decompensated cirrhotics.
Figure 1.
Mean plasma concentration vs. time profiles of lignocaine (circles), monoethylglycinexylidide (MEGX) (squares) and glycinexylidide (GX) (triangles) after i.v. injection of 1 mg kg−1 lignocaine. Open and filled symbols indicate coadministration of placebo or erythromycin, respectively. Standard errors are not shown, for sake of clarity. MEGX and GX concentration data are reported from the first measurable concentration.
Table 3.
Effect of erythromycin coadministration on the pharmacokinetic parameters of monoethylglycinexylidide (MEGX) and glycinexylidide (GX).
| Patients with cirrhosis | ||||||
|---|---|---|---|---|---|---|
| Healthy subjects | Child's class A | Child's class C | ||||
| Parameters | Placebo | Erythromycin | Placebo | Erythromycin | Placebo | Erythromycin |
| MEGX | ||||||
| AUC0, 12 (ng ml, 1 h) | 665 ± 270 | 886 ± 498* | 312 ± 86††† | 405 ± 123*††† | 281 ± 119††† | 347 ± 115*††† |
| Ratio % (95% CI) | 129 (102, 156) | 129 (102, 156) | 127 (110, 144) | |||
| Cmax (ng ml, 1) | 108 ± 64 | 141 ± 114 | 95 ± 79 | 95 ± 73 | 80 ± 69 | 73 ± 67 |
| Ratio 5 (95%CI) | 130 (80, 180) | 110 (62, 158) | 107 (85, 130) | |||
| tmax (h)‡ | 1 (0.25, 2.5) | 1.5 (0.25, 8) | 1 (0.25, 6) | 1 (0.25, 4) | 7 (0.5, 10)† | 8 (2.5, 10)† |
| Ratio % (95% CI) | 139 (31, 247) | 121 (69, 173) | 132 (85, 180) | |||
| GX | ||||||
| AUC0, 12 (ng ml, 1 h) | 315 ± 170 | 233 ± 110 | 136 ± 90†† | 80 ± 65†† | 104 ± 70†† | 95 ± 60†† |
| Ratio % (95% CI) | 80 (45, 115) | 80 (49, 111) | 95 (49, 141) | |||
| Cmax (ng ml, 1) | 71 ± 61 | 62 ± 50 | 46 ± 35 | 36 ± 26 | 21 ± 18 | 28 ± 24 |
| Ratio % (95% CI) | 100 (50, 150) | 71 (34, 107) | 160 (95, 225) | |||
| tmax (h)‡ | 6 (1, 10) | 8 (6, 10) | 7 (1.5, 10) | 8 (6, 10) | 9 (1, 10) | 10 (8, 10) |
| Ratio % (95% CI) | 129 (100, 157) | 142 (30, 255) | 163 (74, 252) | |||
Data are presented as means (s.d.).
P < 0.05 vs. placebo.
P < 0.05
P < 0.01
P < 0.001 vs. healthy volunteers.
Median value (range).
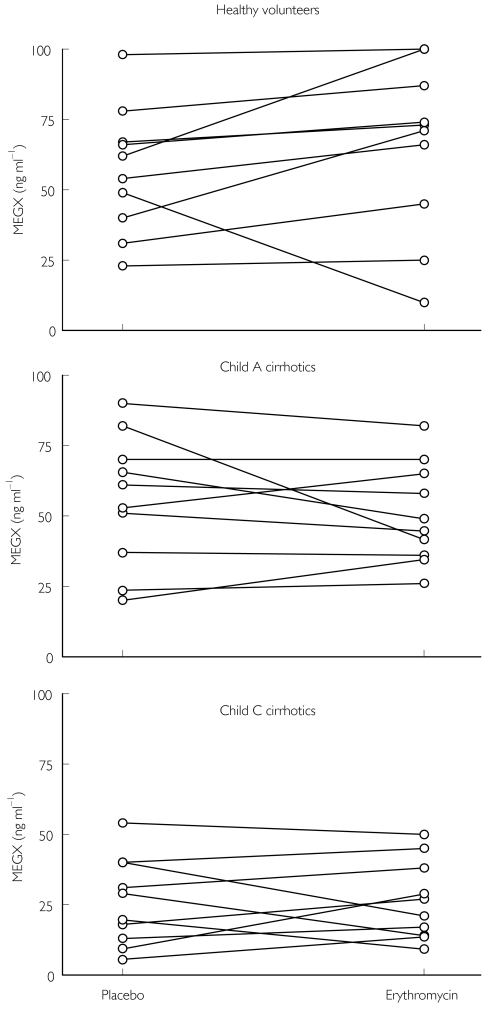
Irrespective of sampling time (15, 30, 45 or 60 min), erythromycin coadministration had no consistent effect on MEGX concentrations (the MEGX liver function test) (P > 0.05 for all paired comparisons) (Figure 2).
Figure 2.
Effect of erythromycin on individual monoethylglycinexylidide (MEGX) plasma concentrations 30 min after i.v. lignocaine injection
Discussion
At variance with previous investigations [5, 6], this study has shown that concomitant administration of erythromycin decreases lignocaine clearance in healthy volunteers to a statistically significant extent. A greater increase in terminal half-life (30%, compared with the 16% reported in [5]) was also observed. Unlike that on other CYP3A4 substrates [3, 4], the modest effect of erythromycin on lignocaine disposition may be due to the following reasons. (i) Lignocaine has a high extraction ratio (62–81%[1]), therefore, its systemic clearance depends more on liver blood flow than metabolic capacity and, consequently, may not be very sensitive to the action of metabolic inhibitors. (ii) Other CYP isoforms may contribute to lignocaine biotransformation. According to a recent report [17], CYP1A2 catalyses the 3-hydroxylation of lignocaine and is also involved in its de-ethylation (MEGX formation), although the relative contribution of this isoform to the metabolic clearance of lignocaine has not been quantified.
In accordance with Isohanni et al.[5], we found that erythromycin coadministration increased MEGX AUC. To explain this observation, Isohanni et al.[5] hypothesized that erythromycin inhibits further MEGX metabolism. Three metabolic routes have been identified for MEGX elimination: 3-hydroxylation, further de-ethylation to GX, and hydrolysis of the amide bond with generation of 2,6-xylidine. However, it has been shown that 3-hydroxy-MEGX production is negligible in man (0.3% of a lignocaine dose [18]). Inhibition of MEGX hydrolysis seems unlikely as, to our knowledge, no effect of erythromycin on amidases has ever been reported. In order to test whether the effect of erythromycin on MEGX concentrations is due to inhibition of MEGX de-ethylation, we also measured GX concentrations. Although the latter were found to be lower after erythromycin coadministration, differences did not reach statistical significance. However, because of the unexpectedly large variability in GX concentrations (Table 3), no definitive conclusion can be drawn from these results. A retrospective power analysis based on the observed differences revealed that more than 20 subjects would have been necessary to demonstrate statistical significance with a power of 0.8. In in vitro experiments with human liver microsomes, we have observed a marked inhibition of MEGX conversion to GX by the structurally related CYP3A4 inhibitor troleandomycin (paper in preparation).
Liver cirrhosis causes progressive alteration of hepatic microcirculation, leading to capillarization of the sinusoids and consequent decrease in drug uptake. Thus, as liver function worsens, the clearance of highly extracted drugs progressively loses its flow-dependence and becomes capacity-limited, as shown for lignocaine [7]. Hence, inhibition of lignocaine metabolizing enzymes should result in a more marked reduction of clearance in patients with liver cirrhosis. Contrary to this hypothesis, erythromycin caused very similar changes in lignocaine clearance in healthy volunteers and the two groups of cirrhotic patients. The lack of a more marked reduction in clearance in cirrhotic patients may be due to reduced uptake of the inhibitory drug by the cirrhotic liver, resulting in decreased enzyme inhibition. In this context, decreased or absent enzyme induction has been observed in liver disease (see [19] and [20] for reviews). Alternatively, a macrolide antibiotic such as erythromycin may not be the ideal drug to test this hypothesis, as its inhibitory action is consequent upon the formation of a nitrosoalkane metabolite [4], which might be produced to a lesser extent in subjects with liver dysfunction. On the other hand, the other currently used CYP3A4 inhibitors, the azole antifungals, have been shown to have no effect on lignocaine and MEGX kinetics [5]. Further tests with highly extracted drugs metabolized by other CYP isoforms are necessary for a definitive assessment of whether or not the clearance of flow-dependent drugs becomes more sensitive to enzyme inhibition in liver cirrhosis.
Although many drugs can induce or inhibit CYP3A4 activity [13], few studies have investigated the possible interference of such drugs with the MEGX test [6, 21–23]. We investigated the effect of erythromycin coadministration on the MEGX test at various sampling times used by others, namely, from 15 to 60 min (see [24] and references therein). In accordance with the results of Swart et al.[6] and those obtained with other known modifiers of CYP3A4 activity [21–23], we found no significant effect of erythromycin on the MEGX test in any of our study groups.
With regard to the effect of liver dysfunction on lignocaine disposition kinetics, this study has shown that the extent of the kinetic modifications is closely dependent on the stage of liver cirrhosis. No significant modifications in CL or t1/2 were observed in patients with compensated (Child's grade A) liver cirrhosis, whereas profound alterations of both parameters were found in decompensated (Child's class C) cirrhotic patients. At variance with the changes in these two parameters, Vss also increased to a significant extent in compensated patients. No such modification had been reported in a previous study [10]; but our results are in good agreement with the observation of Barry et al.[25] that the concentration of α1-acid glycoprotein and, consequently, lignocaine plasma protein binding, start to decrease significantly in Child's grade A liver cirrhosis. Collectively, these findings indicate that only in patients with decompensated liver cirrhosis is a reduction of lignocaine dose required.
In conclusion, unlike previous investigations [5, 6], this study has shown that concomitant administration of erythromycin causes a moderate but statistically significant decrease in lignocaine clearance that, contrary to predictions, is quantitatively similar in healthy subjects and cirrhotic patients. Because erythromycin also significantly increases the AUC of MEGX, which has been shown to have 80–90% of the antiarrhythmic potency of lignocaine [1], maintenance of the rate of lignocaine infusion at the lower end of the therapeutic range (1-4 mg kg−1) may be prudent and it may be advisable to monitor closely patients in the event that inadequate clinical response requires upward dose adjustment. Clearly, only a pharmacodynamic analysis of the erythromycin-lignocaine interaction, following lignocaine infusion to steady state, can provide a definitive answer to this question.
An additional finding of this study is that erythromycin had virtually no effect on MEGX concentrations in the early period after lignocaine injection, and therefore does not interfere with the MEGX test. As analogous observations have been made in patients treated with therapeutic doses of other CYP3A4 inhibitors or inducers [21–23], it may be concluded that the MEGX test is insensitive to the action of modifiers of CYP3A4 activity.
Acknowledgments
This work was supported by a grant from MURST (Ministero per l'Università e la Ricerca Scientifica e Tecnologica).
References
- 1.Pieper JA, Lima H. Lidocaine. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied PharmacokineticsPrinciples of Therapeutic Drug Monitoring. 3. Vancouver, WA: Applied Therapeutics, Inc; 1992. chapter 21. [Google Scholar]
- 2.Bargetzi MJ, Aoyama T, Gonzales FJ, Meyer UA. Lidocaine metabolism in human liver microsomes by cytochrome P450 IIIA4. Clin Pharmacol Ther. 1989;46:521–527. doi: 10.1038/clpt.1989.180. [DOI] [PubMed] [Google Scholar]
- 3.Pai MP, Graci DM, Amsden GW. Macrolide drug interactions: an update. Ann Pharmacother. 2000;34:495–513. doi: 10.1345/aph.19138. [DOI] [PubMed] [Google Scholar]
- 4.Westphal JF. Macrolide-induced clinically relevant drug interactions with cytochrome P-450 (CYP) 3A4: an update focused on clarithromycin, azithromycin and dirithromycin. Br J Clin Pharmacol. 2000;50:285–295. doi: 10.1046/j.1365-2125.2000.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Isohanni MH, Neuvonen PJ, Palkama VJ, Olkkola KT. Effect of erythromycin and itraconazole on the pharmacokinetics of intravenous lidocaine. Eur J Clin Pharmacol. 1998;54:561–565. doi: 10.1007/s002280050513. [DOI] [PubMed] [Google Scholar]
- 6.Swart EL, van der Hoven B, Groeneveld ABJ, Touw DJ, Danhof M. Correlation between midazolam and lignocaine pharmacokinetics and MEGX formation in healthy volunteers. Br J Clin Pharmacol. 2002;53:133–139. doi: 10.1046/j.0306-5251.2001.01182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huet PM, Villeneuve JP. Determinants of drug disposition in patients with cirrhosis. Hepatology. 1983;6:913–918. doi: 10.1002/hep.1840030604. [DOI] [PubMed] [Google Scholar]
- 8.Lin JH, Lu AYH. Inhibition and induction of cytochrome P450 and the clinical implications. Clin Pharmacokinet. 1998;35:361–390. doi: 10.2165/00003088-199835050-00003. [DOI] [PubMed] [Google Scholar]
- 9.Shiffman ML, Luketic VA, Sanyal AJ, Thompson EB. Use of hepatic lidocaine metabolism to monitor patients with chronic liver disease. Ther Drug Monit. 1996;18:372–377. doi: 10.1097/00007691-199608000-00010. [DOI] [PubMed] [Google Scholar]
- 10.Villeneuve LP, Thibeault MJ, Ampelas M, et al. Drug disposition in patients with HBsAg-positive chronic liver disease. Dig Dis Sci. 1987;32:710–714. doi: 10.1007/BF01296136. [DOI] [PubMed] [Google Scholar]
- 11.Conn HO. A peek at the Child-Turcotte classification. Hepatology. 1981;6:673–676. doi: 10.1002/hep.1840010617. [DOI] [PubMed] [Google Scholar]
- 12.Phug RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 13.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP 3A. Annu Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 14.Greenblatt DJ, von Moltke LL, Harmatz JS, et al. Inhibition of triazolam clearance by macrolide antimicrobial agents: in vitro correlates and dynamic consequences. Clin Pharmacol Ther. 1998;64:278–285. doi: 10.1016/S0009-9236(98)90176-X. [DOI] [PubMed] [Google Scholar]
- 15.O'Neal CL, Poklis A. Sensitive HPLC for simultaneous quantification of lidocaine and its metabolites monoethylglycinexylidide and glycinexylidide in serum. Clin Chem. 1996;42:330–331. [PubMed] [Google Scholar]
- 16.Wagner JG. Linear pharmacokinetic equations allowing direct calculation of many needed parameters from the coefficients and exponents of polyexponential equations which have been fitted to the data. J Pharmacokin Biopharm. 1976;4:443–467. doi: 10.1007/BF01062831. [DOI] [PubMed] [Google Scholar]
- 17.Wang JS, Backman JT, Taavitsainen P, Neuvonen PJ, Kivistö KT. Involvement of CYP 1A2 and CYP 3A4 in lidocaine N-deethylation and 3-hydroxylation in humans. Drug Metab Dispos. 2000;28:959–965. [PubMed] [Google Scholar]
- 18.Keenaghan JB, Boyes RN. The tissue distribution, metabolism and excretion of lidocaine in rats, guinea pigs, dogs and man. J Pharmacol Exp Ther. 1972;180:454–463. [PubMed] [Google Scholar]
- 19.Hoyumpa AM, Schenker S. Major drug interactions: effect of liver disease, alcohol and malnutrition. Ann Rev Med. 1982;33:113–149. doi: 10.1146/annurev.me.33.020182.000553. [DOI] [PubMed] [Google Scholar]
- 20.Brower KLR, Dukes GE, Powell JR. Influence of liver function on drug disposition. In: Evans WE, Schentag JJ, Jusko WJ, editors. Applied PharmacokineticsPrinciples of Therapeutic Drug Monitoring. 3. Vancouver, WA: Applied Therapeutics, Inc; 1992. chapter 6. [Google Scholar]
- 21.Rossi JS, Schroder TJ, Vine WH, et al. Monoethylglycinexylidide formation in assessing pediatric donor liver function. Ther Drug Monit. 1992;14:452–456. doi: 10.1097/00007691-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sotaniemi EA, Rautio A, Bäckstrom M, Arvela P, Pelkonen O. CYP 3A4 and CYP 2A6 activities marked by the metabolism of lignocaine and coumarin in patients with liver and kidney disease and epileptic patients. Br J Clin Pharmacol. 1995;39:71–76. doi: 10.1111/j.1365-2125.1995.tb04412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reichel C, Skodra T, Nacke A, Spengler U, Sauerbruch T. The lignocaine metabolite (MEGX) liver function test and P-450 induction in humans. Br J Clin Pharmacol. 1998;46:535–539. doi: 10.1046/j.1365-2125.1998.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orlando R, Palatini P. The effect of age on plasma MEGX concentrations. Br J Clin Pharmacol. 1997;44:206–208. doi: 10.1046/j.1365-2125.1997.00651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry M, Keelig PWN, Weir D, Feely J. Severity of cirrhosis and the relationship of α1-acid glycoprotein concentration to plasma protein binding of lidocaine. Clin Pharmacol Ther. 1990;47:366–370. doi: 10.1038/clpt.1990.41. [DOI] [PubMed] [Google Scholar]