Abstract
Aims
Large oral doses of betaine have proved effective in lowering plasma homocysteine in severe hyperhomocysteinaemia. The pharmacokinetic characteristics and metabolism of betaine in humans have not been assessed and drug monitoring for betaine therapy is not available. We studied the pharmacokinetics of betaine and its metabolite dimethylglycine (DMG) in healthy subjects and in three patients with homocystinuria.
Methods
Twelve male volunteers underwent an open-label study. After one single administration of 50 mg betaine kg−1 body weight and during continuous intake of twice daily 50 mg kg−1 body weight, serial blood samples and 24 h urines were collected to determine betaine and DMG plasma concentrations and urinary excretion, respectively. Patients were evaluated after one single dose of betaine.
Results
We found rapid absorption (t1/2,abs 00.28 h, s.d. 0.17) and distribution (t1/2,λ1 00.59 h, s.d. 0.22) of betaine. A Cmax of 0.94 mmol l−1 (s.d. 0.19) was reached after tmax 00.90 h (s.d. 0.33). The elimination half life t1/2,z was 14.38 h (s.d. 7.17). After repeated dosage, t1/2,λ1 (01.77 h, s.d. 0.75) and t1/2,z (41.17 h, s.d. 13.50) increased significantly (95% CI 0.73, 01.64 h and 19.90, 33.70 h, respectively), whereas absorption remained unchanged. DMG concentrations increased significantly after betaine administration and accumulation occurred to the same extent as with betaine. Renal clearance was low and urinary excretion of betaine was equivalent to 4% of the ingested dose. Distribution and elimination kinetics in homocystinuric patients appeared to be accelerated.
Conclusions
Betaine plasma concentrations change rapidly after ingestion. Elimination half-life increased during continuous dosing over 5 days. Betaine is mainly eliminated by metabolism. More pharmacokinetic and pharmacodynamic studies in hyperhomocysteinaemic patients are needed to refine the current treatment with betaine.
Keywords: betaine, homocysteine, homocystinuria, pharmacokinetics
Introduction
Betaine ([2(N,N,N-trimethyl)ammoniumacetate]) is a zwitterionic compound at neutral pH. It occurs naturally in cells exposed to osmotic stress, such as in various plants or in mammalian kidney medulla [1, 2]. Betaine is commonly ingested in unknown amounts through intake of vegetables, cereals, and seafood [3]. More importantly, betaine is an endogenous catabolite of choline [4]. Betaine in mammals has three known functions. Firstly, as an organic osmolyte, it helps to maintain normal cell volume under osmotic stress and can accumulate to molar concentrations [5]. Secondly, it provides protection against protein denaturation and has been termed a ‘chemical chaperone’[6]. Thirdly, betaine is the only molecule besides methylfolate that provides methyl groups for homocysteine remethylation [3]. The cytosolic enzyme betaine: homocysteine methyltransferase (BHMT, EC 2.1.1.5), abundantly expressed in liver and kidney, catalyses this methyl transfer, yielding methionine and dimethylglycine (DMG). The latter is further metabolized to sarcosine and glycine.
Betaine gains further importance as methyl donor in pathological situations associated with hyperhomocysteinaemia. Severe hyperhomocysteinaemia (or homocystinuria) is rare and caused by genetic disruption of either the oxidative breakdown of homocysteine by cystathionine-beta-synthase (CBS), or the remethylation of homocysteine to methionine by methionine synthase (MS). MS uses 5-methyltetrahydrofolate as cosubstrate that is provided by 5,10 methylenetetrahydrofolate reductase (MTHFR). Homocystinuria is associated with damage to the vascular, neural, and skeletal systems, leading to severe disability or death. Treatment aims to decrease the toxic concentrations of homocysteine by dietary intervention, supplementation of cofactors, and provision of betaine to enhance remethylation [7].
However, mild hyperhomocysteinaemia due to nutritional factors, comorbidity with renal disease, or a frequent genetic polymorphism in the MTHFR gene (677T) and concomitant low folate status is a common condition, which has recently been shown to be linked to several multifactorial diseases, such as vascular disease or neural tube defects [reviewed in 8]. Betaine therapy for mild hyperhomocysteinaemia has not been evaluated, but it has proved to be effective for the treatment of severe hyperhomocysteinaemia and has been used successfully since 1952 [9–13]. In spite of the long-term use of betaine, no systematic study of its pharmacokinetics has been undertaken, other than two anecdotal reports [14, 15]. This lack of information is probably due to the absence of a convenient method for the determination of betaine and its metabolite DMG in body fluids. Detailed pharmacokinetic data on betaine are required for the establishment of optimal regimens.
We have previously developed a rapid and reproducible method for the determination of betaine and DMG concentrations in human body fluids [16]. Using this technique, the present study reports the basic pharmacokinetic characteristics of betaine after oral administration.
Methods
Study design
We designed an open-label study in 12 healthy male volunteers, following the Recommendations for Biomedical Research Involving Humans (current revision of the Declaration of Helsinki of the World Medical Assembly). The study was approved by the Ethics Committee of the Heinrich Heine–University, Düsseldorf. Informed consent was obtained from each participant before they entered the study.
Three study periods, A, B and C, were defined. During period A, single-dose pharmacokinetics were determined. Baseline plasma concentrations were obtained after an overnight fast immediately prior to drug intake. All subjects ingested 0.43 mmol kg−1 body weight (50 mg kg−1 body weight) betaine. During the following 24 h, urine was collected and serial plasma samples were obtained at 5, 15, 30 and 45 min, and 1, 1.5, 2, 3 h, 4, 6, 8, 12 and 24 h after betaine intake. After a washout of 7–14 days, study period B followed. This lasted for 5 days and served to evaluate the pharmacokinetics of betaine during multiple dosing. All volunteers received betaine (0.43 mmol kg−1 body weight) every 12 h and venous blood was collected each morning, immediately before drug intake for determination of trough concentrations. Betaine was stopped after the last morning dose of period B. Period C started immediately after period B and involved the collection of urine and plasma samples for 24 h, as in period A. The decline of plasma concentrations was followed for an additional 3 days from plasma samples taken each morning.
Healthy subjects
All subjects were male and their age ranged from 21 to 36 years (mean 28.1 years, s.d. 4.6). The body mass index, defined as body weight divided by the square of height in metres, was between 18.5 and 30 kg m−2. The subjects were in good health, based on medical history and physical examination, including electrocardiogram and standard laboratory tests (haematology, blood chemistry, hepatitis B surface antigen, HIV antigen, and urine analysis) performed within the 2 weeks prior to the study and at the end of period C. There was no history in any subject, or signs of prevailing active diseases, allergies or hypersensitivities, alcohol or drug exposure. The subjects were not allowed to consume alcohol, nicotine, caffeine or any drugs, and were advised not to undertake physical exercise or major dietary changes during the entire study period.
Patients
Two siblings, a boy and his older sister (patients 1 and 2), with severe hyperhomocysteinaemia and homocystinuria due to MTHFR deficiency were evaluated during reintroduction of betaine therapy after an accidental betaine-free interval of 1 year. Another female patient with classical homocystinuria due to pyridoxine nonresponsive CBS deficiency was also included in the study (patient 3). She had been prescribed a continuous betaine supplement, but voluntarily discontinued drug intake 8 days before the study period. All three patients followed the period A study protocol, but without urine collection. All were in good health during the study and they or their parents, respectively, gave informed consent to participation in the study.
Drug administration
Anhydrous betaine powder (Cystadane™) was kindly provided by Orphan-Europe, Paris. A dose of 0.43 mmol kg−1 body weight (50 mg kg−1 body weight) was dissolved in mineral water and swallowed immediately.
Sampling and drug analysis
Each 4-ml sample of blood was collected in appropriate vacutainers (Becton-Dickinson), anticoagulated with 5 nm K3-EDTA and centrifuged immediately after venepuncture at 15000 g for 15 min at 4 °C. The supernatant plasma was kept frozen below −20 °C for less than 10 days until analysis.
Betaine and DMG plasma concentrations were determined as previously described [16]. Briefly, plasma was derivatized to produce phenacyl esters of the methylamines and compounds were analysed by an isocratic h.p.l.c. procedure with u.v. detection. This method has been shown have limits of detection of 0.005 mmol l−1 for betaine and 0.002 mmol l−1 for DMG. Inter-assay variability was low with coefficients of variation (CV) of 1.3–5.3% for betaine and of 2.0–4.4% for DMG, respectively, in both blood and urine. Intra-assay variability showed a CV of 0.4–3.8% for betaine and of 0.9–2.2% for DMG, respectively, in blood and urine [16].
Pharmacokinetic and statistical analysis
Pharmacokinetic parameters were estimated using a standard software package (TopFit 2.1: Pharmacokinetic and pharmacodynamic data analysis system for the PC; G. Fischer, Stuttgart, Jena, New York 1993). The peak plasma concentration (Cmax) and the time to reach the peak concentration (tmax) were derived directly from the plasma concentration time data. The area under the plasma concentration vs time curve from the time of dosing to the time of the last quantified concentration (AUC(0,24 h)) was computed by the linear trapezoidal method. The theoretical accumulation ratio was calculated as 1/(1-e–λ1×τ), where λ1 denotes the rate constant for the elimination phase derived from period A, where τ was the dosing interval. The following kinetic parameters were estimated using TopFit: Half-life parameters for absorption (t1/2,abs), distribution (t1/2,λ1), and elimination (t1/2,z); the area under the plasma-concentration-time course extrapolated to infinity towards the betaine baseline level (AUC(0,∞); and the mean residence time (MRT). A standard linear two-compartment disposition model for oral drug application with first order absorption kinetics, including a lag period, fitted best to the data. Maximal apparent total plasma clearance (CL/F) was calculated as dose divided by AUC(0,∞) assuming an oral bioavailability of 100%. The maximal apparent volume of distribution at steady state after a single dose was estimated using the formula Vss/F = CL/F× MRT. Renal clearance (CLR) was evaluated as ratio of 24 h urinary excretion to the respective AUC(0,24 h).
Statistical evaluation was performed using standard software. Data were expressed as mean and standard deviation. Differences between kinetic parameters after the first single dose (period A) and the last single dose after multiple dosing (period C) were compared using Student's t-test for paired samples. Differences were considered significant if P values were less than 0.05.
Results
Healthy volunteers
Betaine was well tolerated over the whole experimental period. No adverse events were observed. All routine laboratory data remained unchanged throughout the study.
Baseline concentrations of betaine
Mean ± s.d. baseline concentrations of betaine were 0.032 ± 0.006 mmol l−1 in period A and 0.034 ± 0.009 mmol l−1 in period B, and were not significantly different. The concentrations were 0.075 ± 0.027 mmol l−1 after 24 h during period A, and to 0.104 ± 0.102 mmol l−1 96 h after the last dose in period C.
Plasma pharmacokinetics of betaine
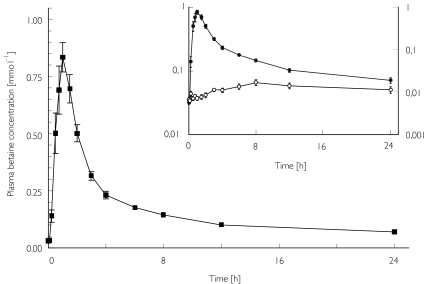
The results for period A are shown in Table 1. Betaine plasma concentrations increased sharply after oral dosing. Betaine was rapidly distributed into a relatively large apparent volume of distribution. The plasma concentration–time curve showed a biexponential decline according to a two-compartment model (Figure 1). Elimination of the drug was rather slow.
Table 1.
Pharmacokinetic parameters after a single dose of betaine in 12 healthy subjects.
| Parameter | Mean± s.d. | Range |
|---|---|---|
| Cmax (data) (mmo l−1) | 0.939 ± 0.194 | 0.663–1.300 |
| Cmax (model) (mmol l−1) | 0.906 ± 0.191 | 0.652–1.320 |
| tmax (data) (h) | 0.90 ± 0.33 | 0.50–1.50 |
| tmax (model) (h) | 0.92 ± 0.29 | 0.52–1.38 |
| AUC(0, 24 h) (mmol l−1 h) | 3.974 ± 0.731 | 2.747–5.240 |
| AUC(0, ∞) (mmol l−1 h) | 5.518 ± 1.919 | 3.730–11.000 |
| tlag (h) | 0.43 ± 0.19 | 0.21–0.72 |
| t1/2,abs (h) | 0.28 ± 0.17 | 0.09–0.61 |
| t1/2,λ1 (h) | 0.59 ± 0.22 | 0.35–1.01 |
| t1/2,z (h) | 14.38 ± 7.17 | 6.04–31.64 |
| MRT (h) | 17.50 ± 9.26 | 7.19–40.90 |
| CL/F* (l h−1 kg−1) | 0.084 ± 0.021 | 0.039–0.115 |
| VSS/F* (l kg−1) | 1.324 ± 0.382 | 0.728–1.896 |
Cmax, maximum plasma concentrations; tmax, times of Cmax; AUC, areas under the plasma concentration/time curve; tlag, lag time; t1/2,abs, half-life of absorption; t1/2, λ1, half-life of distribution; t1/2,z, half-life of elimination; MRT, Mean residence time; CL/F, total oral plasma drug clearance; VSS/F, volume of distribution at steady-state after oral application.
Assuming 100% bioavailability.
Figure 1.
The mean ± s.e.mean betaine plasma concentration vs time profile following a single oral dose of 50 mg kg−1 in 12 healthy subjects. Insert: semilogarithmic plot of mean betaine (•) and DMG (○) plasma concentrations.
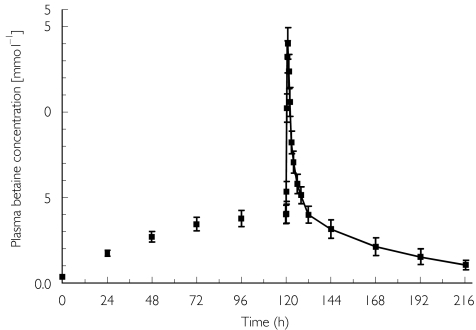
The pharmacokinetics after multiple doses are shown in Figure 2 and Table 2. Whereas tmax and t1/2,abs remained unchanged, Cmax, AUC(0,24 h), t1/2,λ1, and t1/2,z increased significantly after multiple doses (P < 0.01). The mean experimental accumulation ratio clearly exceeded the theoretical accumulation ratio.
Figure 2.
The mean ± s.e.mean betaine plasma concentration vs time profile after multiple dosing of 50 mg kg−1 to 12 healthy volunteers.
Table 2.
Pharmacokinetic parameters after multiple dosing of betaine in 12 healthy subjects (values were evaluated for the time 0–96 h after the final dose (period C).
| Parameter | Mean ± s.d. | Range | 95% CI Δ | P |
|---|---|---|---|---|
| Cmax (data) (mmol l−1) | 1.456 ± 0.308 | 1.050–2.178 | 0.369–0.665 | <0.01 |
| tmax (data) (h) | 0.90 ± 0.25 | 0.34–1.00 | −0.19–0.18 | 0.97 |
| AUC(0,24 h) (mmol l−1 h) | 12.528 ± 4.496 | 8.134–24.118 | 6.244–10.862 | <0.01 |
| AUC(0,96 h) (mmol l−1 h) | 26.298 ± 15.664 | 14.918–71.403 | ||
| tlag (h) | 0.36 ± 0.10 | 0.21–0.48 | −0.20–0.05 | 0.27 |
| t1/2,abs (h) | 0.68 ± 0.25 | 0.32–1.06 | −0.07–0.21 | 0.35 |
| t1/2, λ1 (h) | 1.77 ± 0.75 | 0.60–2.68 | 0.73–1.64 | <0.01 |
| t1/2,z (h) | 41.17 ± 13.50 | 27.81–74.32 | 19.89–33.70 | <0.01 |
| Accumulation ratio (theoretetical) | 2.29 ± 0.84 | 1.34–4.33 | ||
| Accumulation ratio (experimental) | 3.14 ± 0.80 | 2.22–4.60 |
Cmax, maximum plasma concentrations; tmax, times of Cmax; AUC, areas under the plasma concentration/time curve; tlag, lag time; t1/2abs, half-life of absorption; t1/2,λ1, half-life of distribution; t1/2,z, half-life of elimination. 95% confidence intervals and P values are given for differences between results of period A and period C.
Urinary excretion of betaine
Baseline 24 h excretion of betaine before drug administration was measured in only four individuals and gave a mean value 0.159 ± 0.103 mmol. Table 3 shows the results for renal betaine excretion after one single dose in period A and C, respectively. Betaine excretion was low in period A and increased threefold after multiple doses. Renal clearance was only 5.3% of apparent total plasma clearance in period A and remained essentially the same during the study period.
Table 3.
Urinary excretion of betaine after a single dose (period A) or multiple doses (period C) of oral betaine in 12 healthy subjects.
| Parameter | Mean ± s.d. | Range |
|---|---|---|
| Period A | ||
| 24 h urinary excretion (mmol) | 1.338 ± 1.111 | 0.153–4.324 |
| % of oral dose (%) | 4.0 ± 3.4 | ;0.4–13.1 |
| CLR (l h−1 kg−1) | 0.0044 ± 0.0037 | 0.0004–0.0139 |
| % of total plasma CL (%) | 5.3 ± 3.9 | 0.5–14.3 |
| Period C | ||
| 24 h urinary excretion (mmol) | 4.361 ± 2.105 | 0.756–7.444 |
| CLR (l h−1 kg−1) | 0.0045 ± 0.0022 | 0.0006–0.0086 |
CLR, renal clearance.
Plasma pharmacokinetics of DMG
The mean ± s.d. initial concentrations of DMG were 0.007 ± 0.003 mmol l−1 for period A and 0.008 ± 0.005 mmol l−1 for period B. DMG rose significantly after 3 h, to a peak concentration of 0.019 ± 0.008 mmol l−1 at tmax 09.72 h ± 7.24 in period A. During periods B and C, DMG concentration rose significantly by 0.033 mmol l−1 (95% CI 0.017, 0.049) to a peak concentration Cmax of 0.052 ± 0.034 mmol l−1 after 126 ± 5 h, corresponding to 06.45 h after the last betaine dose. The AUC(0,24 h) for period A was 0.308 ± 0.147 mmol l−1 h, whereas the AUC(0,24 h) for period C was 0.999 ± 0.662 mmol l−1 h. The accumulation ratio of 3.31 ± 1.25 was comparable with that of betaine.
Urinary excretion of DMG
The mean 24 h urinary excretion of DMG after single dose (period A) was 0.339 ± 0.362 mmol (range 0.055–1.253). After multiple dosage (period C), the 24 h excretion of DMG in urine rose significantly by 1.079 mmol (95% CI 0.543, 1.616) to 1.407 ± 1.150 mmol (range 0.252–4.139), compared with period A. The renal clearance of DMG was calculated as 0.014 ± 0.018 l h−1 kg−1 (range 0.004–0.017) for period A and 0.017 ± 0.007 l h−1 kg−1 (range 0.005–0.027) for period C (P > 0.05).
Patients with homocystinuria
Baseline concentrations of betaine and DMG
Baseline concentrations of betaine were 0.004, 0.012, and 0.011 mmol l−1 in patients 1, 2, and 3, respectively, before reintroduction of betaine. Betaine concentrations were 0.006 and 0.031 mmol l−1 after 24 h, 0.003 and 0.012 mmol l−1 after 48 h in patients 1 and 2, respectively. Patient 3 had a betaine concentration of 0.050 mmol l−1 24 h after dosing. DMG baseline concentrations were below the detection limit (<0.001 mmol l−1) in all three patients.
Plasma pharmacokinetics of betaine
Absorption was rapid in patients 1 and 3, whereas patient 2 had a much longer t1/2,abs than all the healthy volunteers and the two other patients (Table 4). Distribution and elimination half-lives were somewhat shorter in patients 1 and 3 and apparent total plasma clearance appeared to be increased in all three patients compared with the healthy volunteers.
Table 4.
Pharmacokinetic parameters after a single dose of betaine in patients with homocystinuria
| Parameter | Patient 1 | Patient 2 | Patient 3 |
|---|---|---|---|
| Age (years) | 5.8 | 10.2 | 25.5 |
| Body weight (kg) | 22 | 40 | 91 |
| Height (cm) | 114 | 142 | 183 |
| Cmax (data) (mmol l−1) | 0.506 | 0.556 | 1.176 |
| Cmax (model) (mmol l−1) | 0.466 | 0.506 | 1.070 |
| tmax (data) (h) | 1.20 | 2.00 | 0.88 |
| tmax (model) (h) | 1.00 | 2.06 | 0.93 |
| AUC(0, 24 h) (mmol l−1 h) | 1.321 | 3.491 | 4.215 |
| AUC(0, ∞) (mmol l−l h) | 1.450 | 4.230 | 4.810 |
| t1/2,abs (h) | 0.46 | 1.22 | 0.37 |
| t1/2,λ1 (h) | 0.46 | 1.22 | 0.37 |
| t1/2,z (h) | 6.63 | 23.23 | 9.70 |
| MRT (h) | 5.81 | 18.70 | 11.10 |
| CL/F* (l h−1 kg−1) | 0.297 | 0.102 | 0.089 |
| VSS/F* (l kg−1) | 1.726 | 1.907 | 0.992 |
Cmax, maximum plasma concentrations; tmax, times of Cmax; AUC, areas under the plasma concentration/time curve; t1/2;λ1, half,life of absorption; t1/2;λ1, half,life of distribution; t1/2;z, half,life of elimination; MRT, Mean residence time; CL/F, total oral plasma drug clearance; VSS/F, volume of distribution at steady,state after oral application.
Assuming 100% bioavailability.
Plasma kinetics of DMG
DMG rose to a mean peak concentration of 0.011 and 0.010 mmol l−1 4–5 h after administration of betaine in patients 1 and 2, respectively. In patient 3, DMG rose to a maximum of 0.140 mmol l−1 after 6 h. Twenty-four hours after the dose, plasma concentrations returned to their initial values in patients 1 and 2, whereas in patient 3 DMG remained elevated at 0.102 mmol l−1.
Discussion
Despite its long-standing use for treatment of severe hyperhomocysteinaemia, the pharmacokinetics of betaine have not been explored thoroughly in animals or humans. Previously published values of plasma betaine concentrations in blood samples taken randomly from healthy individuals show similar results to our study. Allen and coworkers [17] found serum concentrations of 0.046 ± 0.014 mmol l−1 (n = 60), which were not affected by age and gender. One group [18] found mean plasma concentrations of 0.034 ± 0.011 mmol l−1 in females (n = 37), and of 0.047 ± 0.018 mmol l−1 in males (n = 35). In a previous study [16], we reported a mean plasma concentration of 0.027 mmol l−1 (n = 12). In the present study our baseline concentrations are consistent with these previous findings.
After oral uptake of 0.43 mmol kg−1 body weight betaine in 12 healthy volunteers, betaine was rapidly absorbed with a sharp plasma concentration peak. Distribution half-life was short after the first dose. Assuming 100% oral bioavailability, a relatively high apparent distribution volume of 1.32 l kg−1 was calculated, indicating extensive uptake of betaine into tissue. Of more relevance for the use of betaine in hyperhomocysteinemia, its pharmacokinetics were also studied after a therapeutic repeated dose regimen of 0.43 mmol kg−1 every 12 h for 5 days, with a final single dose on day 6. Whereas absorption kinetics did not change over this period, distribution half-life was significantly longer compared with that after single dose, indicating that transport and redistribution between different compartments might have been partially saturated.
Betaine is exclusively and irreversibly catabolized by the zinc metalloenzyme betaine: homocysteine methyltransferase (BHMT) [20]. The accumulation of betaine during the 5-day study period B and the increased elimination half-life measured in period C indicate limited capacity for betaine metabolism as a result of either saturation or inhibition.
The Km of BHMT for betaine metabolism is 2.2 mmol l−1[21]. Given that betaine is known to be present in liver and kidney and because it fits the high apparent volume of distribution, a concentration of betaine in hepatocytes in the molar range and thus near to or even above the Km of BHMT can be expected, and would be compatible with saturation of metabolism.
We also found a considerable increase in DMG plasma concentration and urinary excretion during multiple betaine dosing. This provides evidence for an enhancement of betaine metabolism by exogenous betaine in healthy subjects, which has also been suggested by others [22]. In contrast, it has been shown that DMG inhibits BHMT activity in vitro with a low Ki of approximately 0.010 mmol l−1[17]. Increasing concentrations of DMG during periods B and C of the study could have led to increased inhibitory effects on BHMT activity.
The mean ratio between AUC(0,24 h) of betaine and DMG in period A was 12.90 compared with 12.54 for period C. Thus, both betaine and DMG appear to accumulate to the same extent. This observation argues against saturation of the BHMT-mediated reaction as the sole cause for the increased elimination half-life of betaine. Taking into account an unchanged renal clearance and the plasma accumulation of DMG, our data provide also evidence for a limited capacity of healthy subjects to metabolize DMG by oxidative demethylation to sarcosine, which seems to be the rate-limiting step in betaine metabolism and might indirectly influence the latter through inhibition of BHMT by DMG. In conclusion, increased DMG formation and saturation of DMG metabolism with subsequent product inhibition of BHMT would best explain our results.
Increasing availability of betaine enhances metabolic flow through BHMT until saturation occurs. Animal studies have shown that enzyme induction can then occur, particularly when methionine availability is low [20]. All subjects in the present study were on normal western diets with a high protein content, when methionine availability should be high. BHMT induction should therefore be negligible in this study.
Betaine is not a xenobiotic but an endogenous metabolite of choline. In an attempt to correct for the contribution of endogenous betaine, we subtracted the baseline plasma betaine pool, defined by the product of betaine concentrations at t0 multiplied by the study period observation, from total AUC(0, 24 h). The same percentage of total area was subtracted from AUC(0, ∞). We observed a decrease in AUC(0, 24 h) of 19% and a corresponding increase in total plasma clearance and distribution volume of 24% after correction in period A. We consider this to be a minor confounder in the interpretation of results and did not perform such a correction for period C or on the patient data, where baseline betaine plasma pools were even smaller relative to the AUC after betaine dosage.
Betaine normally accumulates in human kidney medulla [2]. Its release into urine is dramatically augmented after water diuresis [5]. There is evidence that plasma concentrations are under homeostatic control, and the urinary excretion of betaine shows marked variability. Betaine transporter gene (BGT1) expression is directly regulated by tonicity of the intracellular fluid [19], therefore different intakes of water and salts during the study could account for some of the interindividual variability in the elimination kinetic data. However, renal clearance of betaine did not change during the study and contributed to only a minor extent to the elimination of betaine. A fractional clearance of less than 6% in male healthy subjects has been demonstrated for betaine [17, 18]. In the present study, renal clearance corresponded to 5.3% of total oral plasma clearance and 4.0% of the ingested dose was excreted as betaine in the 24-h urine samples during period A.
Betaine baseline concentrations in the three patients with severe hyperhomocysteinaemia before reintroduction of betaine therapy were decreased compared with healthy subjects. Plasma betaine concentrations in patients undergoing continuous high-dose betaine treatment range from 0.02 to 2.68 mmol l−1[14, 17]. Obviously, concentrations depend heavily on the interval between dosing and time of measurement. Betaine kinetics after the first dose in the three patients were similar to those in healthy volunteers in period A. The results of patient 2 were distorted by delayed absorption. Distribution and elimination half-lives of betaine in patients 1 and 3 were decreased and total plasma clearance increased in all three individuals compared with healthy subjects. The apparent volume of distribution was increased in patients 1 and 2, and the lower value in patient 3 might also relate to their obesity. A short report on betaine kinetics in five homocystinuric patients described absorption half-lives of between 0.3 and 1.4 h and oral total clearances ranging from 0.06 to 0.21 l h−1 kg−1[15], which are comparable with our results. In spite of the small number of observations, the turnover of betaine seems to be accelerated in patients with homocystinuria. From these findings it can be hypothesized that the endogenous betaine pool is diminished in severe hyperhomocysteinaemia, where increased remethylation of homocysteine via BHMT takes place.
The dosing interval selected for the present study (12 h), which corresponds to current practice in the treatment of hyperhomocysteinaemia, appears not to be adequate for maintaining a constant high concentration of betaine in plasma. However, we have no data on intrahepatocytic betaine concentrations, which are likely to be more constant. Our results might provide a basis for further pharmacokinetic and pharmacodynamic studies of betaine in patients with mild or severe hyperhomocysteinaemia with the aim of optimizing treatment modalities.
Acknowledgments
Financial support by Orphan Europe, 60 Avenue du Général De Gaulle, F-92046 Paris la Défense, France, is gratefully acknowledged.
References
- 1.Weretilnyk EA, Hanson AD. Molecular cloning of a plant betaine-aldehyde dehydrogenase, an enzyme implicated in adaptation to salinity and drought. Proc Natl Acad Sci USA. 1990;87:2745–2749. doi: 10.1073/pnas.87.7.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sizeland PCB, Chambers ST, Lever M, Bason LM, Robson RA. Organic osmolytes in human and other mammalian kidneys. Kidney Int. 1993;43:448–453. doi: 10.1038/ki.1993.66. [DOI] [PubMed] [Google Scholar]
- 3.Zeisel SH, Blusztajn JK. Choline and human nutrition. Annu Rev Nutr. 1994;14:269–296. doi: 10.1146/annurev.nu.14.070194.001413. [DOI] [PubMed] [Google Scholar]
- 4.Chern MK, Pietruszko R. Evidence for mitochondrial localization of betaine aldehyde dehydrogenase in rat liver: purification, characterization, and comparison with human cytoplasmic E3 isozyme. Biochem Cell Biol. 1999;77:179–187. [PubMed] [Google Scholar]
- 5.Burg M. Molecular basis of osmotic regulation. Am J Physiol. 1995;268:F983–F996. doi: 10.1152/ajprenal.1995.268.6.F983. [DOI] [PubMed] [Google Scholar]
- 6.Caldas T, Demont-Caulet N, Ghazi A, Richarme G. Thermoprotection by glycine betaine and choline. Microbiology. 1999;145:2543–2548. doi: 10.1099/00221287-145-9-2543. [DOI] [PubMed] [Google Scholar]
- 7.Kang SS. Treatment of hyperhomocystinemia: physiological basis. J Nutr. 1996;126:1273S–5S. doi: 10.1093/jn/126.suppl_4.1273S. [DOI] [PubMed] [Google Scholar]
- 8.Schwahn B, Rozen R. Polymorphisms in the methylenetetrahydrofolate reductase gene. Clinical consequences. Am J Pharmacogenomics. 2001;1:189–201. doi: 10.2165/00129785-200101030-00004. [DOI] [PubMed] [Google Scholar]
- 9.Morrison LM. Results of betaine treatment of atherosclerosis. Am J Dig Dis. 1952;19:381–384. doi: 10.1007/BF02881126. [DOI] [PubMed] [Google Scholar]
- 10.Wendel U, Bremer HJ. Betaine in the treatment of homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. Eur J Pediatr. 1984;142:147–150. doi: 10.1007/BF00445602. [DOI] [PubMed] [Google Scholar]
- 11.Brenton DP, Cusworth DC, Dent CE, Jones EE. Homocystinuria. Clinical and dietary studies. Q J Med. 1966;35:325. [PubMed] [Google Scholar]
- 12.Komrower GM, Sardharwalla IB. The dietary treatment of homocystinuria. In: Carson NAJ, Raine DN, editors. Inherited Disorders Of Sulfur Metabolism. Edinburgh, London: Churchill Livingstone; 1971. pp. 234–263. [Google Scholar]
- 13.Smolin LA, Benevenga NJ, Berlow S. The use of betaine for the treatment of homocystinuria. J Pediatr. 1981;99:467–472. doi: 10.1016/s0022-3476(81)80352-6. [DOI] [PubMed] [Google Scholar]
- 14.Sakura N, Ono H, Nomura S, Ueda H, Fujita N. Betaine dose and treatment intervals in therapy for homocystinuria due to 5,10-methylenetetrahydrofolate reductase deficiency. J Inher Metab Dis. 1998;21:84–85. doi: 10.1023/a:1005331902497. [DOI] [PubMed] [Google Scholar]
- 15.Johnson TN, Rostami-Hodjegan A, Matthews A, Tucker GT, Bonham J. Betaine kinetics in man. Br J Clin Pharmacol. 2001;51:386P–387P. [Google Scholar]
- 16.Laryea MD, Steinhagen F, Pawliczek S, Wendel U. Simple method for the routine determination of betaine and N.N-dimethylglycine in blood and urine. Clin Chem. 1998;44:1937–1941. [PubMed] [Google Scholar]
- 17.Allen RH, Stabler SP, Lindenbaum J. Serum betaine, N,N-dimethylglycine and N-methylglycine levels in patients with cobalamin and folate deficiency and related inborn errors of metabolism. Metabolism. 1993;42:1448–1460. doi: 10.1016/0026-0495(93)90198-w. [DOI] [PubMed] [Google Scholar]
- 18.Lever M, Sizeland PCB, Bason LM, Hayman CM, Chambers ST. Glycine betaine and proline betaine in human blood and urine. Biochim Biophys Acta. 1994;1200:259–264. doi: 10.1016/0304-4165(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 19.Miyakawa H, Rim JS, Handler JS, Kwon HM. Identification of the second tonicity-responsive enhancer for the betaine transporter (BGT1) gene. Biochim Biophys Acta. 1999;1446:359–364. doi: 10.1016/s0167-4781(99)00122-0. [DOI] [PubMed] [Google Scholar]
- 20.Park EI, Garrow TA. Interaction between dietary methionine and methyl donor intake on rat liver betaine-homocysteine methyltransferase gene expression and organization of the human gene. J Biol Chem. 1999;274:7816–7824. doi: 10.1074/jbc.274.12.7816. [DOI] [PubMed] [Google Scholar]
- 21.Millian NS, Garrow TA. Human betaine-homocysteine methyltransferase is a zinc metalloenzyme. Arch Biochem Biophys. 1998;356:93–98. doi: 10.1006/abbi.1998.0757. [DOI] [PubMed] [Google Scholar]
- 22.Storch KJ, Wagner DA, Young VR. Methionine kinetics in adult men: effects of dietary betaine on L-[2H3-methyl-1–13C]-methionine. Am J Nutr. 1991;54:386–394. doi: 10.1093/ajcn/54.2.386. [DOI] [PubMed] [Google Scholar]