Abstract
Studies of novel centrally acting drugs in healthy volunteers are traditionally concerned with kinetics and tolerability, but useful information may also be obtained from biomarkers of clinical endpoints. A useful biomarker should meet the following requirements: a consistent response across studies and drugs; a clear response of the biomarker to a therapeutic dose; a dose–response relationship; a plausible relationship between biomarker, pharmacology and pathogenesis. In the current review, all individual tests found in studies of benzodiazepine agonists registered for anxiety in healthy volunteers since 1966 were progressively evaluated for compliance with these requirements. A MedLine search yielded 56 different studies, investigating the effects of 16 different benzodiazepines on 73 different (variants of) neuropsychological tests, which could be clustered into seven neuropsychological domains. Subjective and objective measures of alertness were most sensitive to benzodiazepines. The most consistent effects were observed on saccadic peak velocity (SPV) and visual analogue scores ( VAS) of alertness, where 100% and 79% of all studies respectively showed statistically significant effects. A dose–response relationship could be constructed for temazepam and SPV, which was used to determine dose equivalencies relative to temazepam, for seven different benzodiazepines. These dose equivalencies correlated with the lowest recommended daily maintenance dose (r2 = 0.737, P < 0.05). This relationship between SPV reduction and clinical efficacy could reflect the clinical practice of aiming for maximum tolerated levels, or it could represent a common basis behind SPV reduction and anxiolytic activity for benzodiazepines (probably sedation). The number of tests used in human psychopharmacology appears to be excessive and their sensitivity and reproducibility low.
Keywords: biomarker, benzodiazepines, cognition, phase I, saccadic eye movement
Introduction
Traditionally, phase 1 studies are mainly concerned with the pharmacokinetics and tolerability of a new drug in healthy volunteers. However, increasing efforts are made to include measures for efficacy as early as in phase 1 studies. This is especially the case for neuropsychiatric disorders where phase 2 studies in patients can be difficult to realize due to practical or ethical issues such as concomitant or previous treatment, adaptation of dose, and the wide variety of types and severity of psychopathology.
Studies in healthy volunteers evade most of the methodological and logistic problems of patient studies, but other complications arise. Most early phase 1 studies are highly dependent on the used biomarker. However, useful information on the potential therapeutic effects of the investigational drug at an early stage could enhance the drug development programme of the new compound.
Although no validated biomarker for anxiolysis exists, in general a useful biomarker for activity of a drug class should meet the following criteria:
a clear, consistent response across studies (from different research groups) and drugs from the same class
a clear response of the biomarker to therapeutic doses
a dose (concentration)-response relationship
a plausible relationship between the biomarker, the pharmacology of the drug class and the pathogenesis of the therapeutic area.
Previously, these criteria were used to evaluate the usefulness of biomarkers for the effects of antipsychotic drugs in healthy volunteers [1]. In the current review, the effects of benzodiazepines in healthy volunteers were evaluated using the same methodology. Benzodiazepines are registered for different indications (like anxiety disorders, epilepsy treatment, insomnia and pre-medication in anaesthesiology), often with various doses and formulations for each indication. To facilitate the review, it was limited to (doses of) benzodiazepines that are registered or investigated for the treatment of anxiety disorders.
Methods
Structured literature evaluation
A broad MedLine search (keywords: (anxiety or anxiolytic) and (model or parameter or effect or *dynamic) and healthy and (subjects or volunteers)) revealed a large number of individual tests, with an apparent lack of standardization between the studies even for the same tests. First, all studies where an anxiolytic benzodiazepine was administered were filtered out. The results of these studies for each individual test, drug and dosage were put into a Microsoft Access® database (97 SR-2; Microsoft Corp., Redmond, USA). Most studies used different tests on different doses of a benzodiazepine, which were all regarded as independent measures of drug effect. The tests could then be roughly divided into neuropsychological/motor skills, subjective assessments, and neurophysiological measurements. This approach allowed the preservation of individual study data in early stages, followed by a progressive condensation of results in logical clusters.
Grouping of individual test results
A structured procedure described previously [1] was adopted in order to obtain an overview. This method includes progressive evaluation of all the reported tests on the basis of the mentioned criteria. The purpose of this review was to identify generally applicable biomarkers of benzodiazepine action. Results from tests that were used only once or by one research group could not be generalized, and were therefore not individually analysed. Such tests were grouped with other comparable tests. The first step in this process included grouping of tests that could be regarded as variants from a basic form (e.g. all tests determining the ability to discriminate flash or flicker frequencies grouped as the test cluster ‘flicker discrimination’). Subsequently, a catalogue of psychological tests [2] was used to group these test clusters further to the neuropsychological domain they actually measure. The results of the effects on these domains were also reviewed.
In most cases, individual test results could not be recorded quantitatively, considering the large diversity of methods, parameters and treatments. Instead, the ability of a test to show a statistically significant difference from placebo or baseline was scored as + (improvement/increase), = (no significant effect) or − (impairment/decrease). Although statistical significance is not only determined by the test variance but also by other factors such as group size, this approach at least allowed an evaluation of the applicability of a test as a biomarker in typical early drug development studies. No efforts were made to further quantify the level of statistical significance at this stage.
Dose normalization
The chance that a test will detect a difference from placebo is expected to increase with dose. To investigate this possibility, it was determined for each individual benzodiazepine and test whether the number of statistically significant results increased with the dose. In this way, the most frequently used tests and drug dosages could be compared for dose-dependency. In many cases however, the number of tests or doses was too small to determine a relationship. To obtain an overview of dose-effects across benzodiazepines, drug dosages were pooled into ‘lower’, ‘medium’ and ‘higher’ dosages. The ‘medium’ dose was determined as the lowest recommended therapeutic dose. The ‘lower’ and ‘higher’ doses were all dosages below or above this level. Benzodiazepines often have different doses for different indications. In such cases, the recommended anxiolytic starting dose was chosen.
This approach allowed the identification of tests showing a consistent response across studies and benzodiazepines and those with a clear response to a therapeutic dose of the anxiolytic (requirements 1 and 2 from the introduction). All measurements fulfilling these criteria were further tested for compliance with requirements 3 and 4: the existence of dose–response relationship and the plausibility of a mechanistic relationship, by reference to the original publications and the neuropharmacological literature. In this case, the original test results were used if possible, rather than statistical significance and effect direction.
Neuropsychological/motor skill tests
In the first phase of the literature review, tests from different studies were only grouped if they were equal as judged from name and description or literature reference [e.g. all Digit Symbol Substitution Tests (DSST)], but all variants or related forms of the tests (DCCT, SDST, etc.) were treated separately.
Next, all tests that could be regarded as variants from a basic form were clustered as indicated in Table 1. Thus, all tests determining the ability to discriminate flash or flicker frequencies were grouped as ‘flicker discrimination’. These data were used to determine the consistency of results within test clusters and to identify potential dose-effects.
Table 1.
Neuropsychological tests reported and clustered with similar tests and the affected domain.
| Test | Cluster | Domain |
|---|---|---|
| Arithmetic addition test | Intelligence | Achievement |
| Differential reinforcement of low response rate | Divided attention | Attention |
| Divided visual attention | ||
| Continuous attention | DSST-like | |
| DSST | ||
| SDST | ||
| Critical flicker fusion | Flicker discrimination | |
| Tone discrimination | ||
| Two flash fusion | ||
| Addition | Other vigilance | |
| Auditory discrimination task | ||
| Binaural stimulation test | ||
| Number of minisleeps | ||
| Number vigilance | ||
| Vigilance | ||
| Visual vigilance | ||
| Card rotations | Complex informationprocessing | Executive |
| Card sorting | ||
| Logical reasoning | ||
| Mean RT signal identification | ||
| Repeated acquisition | ||
| Repeated acquisition (2nd order) | ||
| Sequence completion | ||
| Signal identification | ||
| Subjektieve Leistungseinschätzung | ||
| Prepulse inhibition | Inhibition task | |
| Stroop colour word test | ||
| 15 words test (delayed) | Delayed recall | Memory |
| Auditory recall (delayed) | ||
| Cued recall test | ||
| Long-term visual memory | ||
| Picture recall (delayed) | ||
| Word recall (delayed) | ||
| Word stem completion | ||
| 15 words test (immediate) | Immediate recall | |
| Auditory recall (immediate) | ||
| Immediate visual memory | ||
| Number recognition | ||
| Picture recall | ||
| Picture recognition | ||
| Randt memory test | ||
| Running word recognition | ||
| Verbal memory | ||
| Williams' word memory test | ||
| Word recall (immediate) | ||
| Word recognition | ||
| Memory scanning | Learning | |
| Finger tapping | Manipulation | Motor |
| Anterior tibialis activation latency | Motor control | |
| Body sway | ||
| Functional reach | ||
| Pursuit aiming | ||
| Pursuit rotor | ||
| Subcritical wheel tracking | ||
| Trace sine-wave | ||
| Tracking | ||
| Vvisual motion integration | ||
| Visual tracking task | ||
| Wiener gerät | Hand-eye coordination | Visual, visuomotorand auditory |
| Aerp reaction time | ||
| Auditory reaction time | ||
| Choice reaction time | ||
| Complex choice reaction time | ||
| Reaction time | ||
| Simple choice reaction time | ||
| Simple reaction time | ||
| Sternberg memory test | ||
| Visual reaction time | Reaction time | |
| Bourdon cancellation | Search | |
| Letter cancellation | ||
| Rotated designs matching to sample | ||
| Symbol cancellation | ||
| Visual attention | ||
| Visual search |
Although many different methods are used to evaluate the functional effects of benzodiazepines, most actually measure a limited number of core features. Neuropsychological/motor skills tests can be categorized according to a catalogue of neurocognitive tests (attention, executive, etc.) [2], as presented in Table 1. This catalogue divides tests according to different neuropsychological domains, assuming that the results of each test are mainly (although not exclusively) determined by one of these domains.
Subjective assessments
For the subjective assessments, most individual scales corresponded to ‘alertness’, ‘mood’ and ‘calmness’. These are similar to the scales proposed by Norris [68] and applied to CNS-drug evaluation by Bond & Lader [69]. Other subjective scales could be grouped under ‘craving’, ‘dizziness’, ‘drug effect’, ‘psychomimetic’, ‘sleep’ and ‘symptoms’.
Neurophysiological assessments
Electroencephalography (EEG)
This technique is sensitive to a wide range of centrally active substances, although the exact mechanism is hardly ever known [10–14]. EEG studies differ in numbers of leads, technical settings and EEG-quantification methods, but they usually report effects per EEG-frequency band, which are divided into delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha (7.5–11.5 Hz) and beta (above 11.5 Hz; subdivided into beta 1 (11.5–30Hz) and beta 2 (above 30 Hz) if possible). Results describing the total EEG-spectrum were scored under the cluster EEG.
Eye movements
Smooth pursuit and saccadic eye movements have been frequently used to assess CNS-drug (side)-effects [3–5]. Saccadic eye movements provide information on the sedative properties of benzodiazepines. Although there are different techniques to measure eye movements, most studies report peak velocity for visually guided saccades or sometimes antisaccades (where subjects are instructed to look away from the target). Non- and anti-saccadic movements involve more complex cognitive processing than stimulus-evoked saccades and are considered as a separate cluster. Smooth pursuit eye movements are also treated separately. They are often reported as deviations from the time that the eyes closely followed the target. Eye blink is the cluster containing tests concerning spontaneous eye blinking. Dopaminergic pathways are thought to be involved in spontaneous eye blinking [6–8]. Startle eye blinks can be elicited by sudden noise bursts. They are part of the polysynaptic startle reflex and occur involuntarily as fast as 20–150 ms after stimulus onset. The tests clustered under ‘startle reflex’ were ‘startle blink’ and ‘acoustic startle’.
Analysis of relationship with therapeutic efficacy and in vitro pharmacology
Biomarkers that complied with the first three mentioned criteria were subsequently evaluated for potential relationships between the biomarker and the therapeutic effects of the drugs. Establishing such relationships would require clear dose–response relationships for each drug, to determine potency measures for the biomarker and therapeutic effects. For the validation of the biomarker finding a close relationship between the potency of the drug to show an effect on this biomarker and the therapeutic doses would be extremely valuable. Establishing this relationship is only possible with well-defined potencies that affect the biomarker determined from dose–response relationships for each benzodiazepine. For most benzodiazepines this relationship was not provided by the literature. As an alternative approach, a reference curve was constructed for each of the biomarkers, using quantitative results from the most frequently used benzodiazepine. Next, the potencies of other benzodiazepines were expressed relative to this reference agent, by plotting the observed effect of the benzodiazepine on the curve and determining the corresponding dose of the reference drug. Benzodiazepine dosages that caused a larger response than observed with the reference drug were not plotted on the reference curve, i.e. data were not extrapolated beyond the extent of the curve. In this way, for each benzodiazepine dose an equipotent reference drug dose was determined that would theoretically cause a similar response. Subsequently, the mean of these values was calculated per benzodiazepine. Comparing these mean biomarker-affecting potencies with the lowest recommended daily therapeutic maintenance dose was the next step in examining the value of a biomarker for predicting the eventual therapeutic efficacy. Finally, the mean biomarker-affecting potencies were plotted against in vitro Kd affinities for the benzodiazepine binding site [9, 10] to evaluate the relationship between the biomarker and the in vitro pharmacology of the drugs. This investigation of a plausible relationship between the biomarker, the pharmacology of the drug class and the pathogenesis of the therapeutic area (the last defined requirement of a useful biomarker) was performed using data from studies that include effects of drugs from the benzodiazepine class irrespective of their registered indication.
Dose–response reference curves could only be constructed if, for a particular test (cluster) enough quantifiable data were available for a single benzodiazepine. Often, the number of studies with the potential reference drug was too low, or the presentation of results too variable. In these cases, doses of different benzodiazepines were represented (‘normalized’) as fractions of the medium therapeutic dose. Similarly quantified test results were plotted against these ‘normalized’ doses, to identify relationships between the biomarker and the therapeutic (anxiolytic) benzodiazepine doses.
Results
The literature search yielded 56 different studies using 16 different benzodiazepines, published since 1966 [14–67]. There were 173 different tests used, on average 3.1 tests per study. On average 20 subjects participated in each study (range 4–145 subjects). On average 1.2 doses were given per study. All reported psychological tests and the relevant clusters and psychological domains are represented in Table 1. The benzodiazepines reported in the reviewed articles are listed in Table 2 with the therapeutic dose ranges for the various routes of administration. Fifty-eight tests that never showed any significant effect are listed in Table 3.
Table 2.
Benzodiazepines reported in the reviewed articles, therapeutic dose ranges, dissociation constants at benzodiazepine binding site and SPV dose equivalences (see text for explanation).
| Drug | Route | Lowest therapeuticdose (mg) | Highest therapeuticdose (mg) | Kdat benzodiazepinesite (nm) | SPV dose equivalences(10 mg temazepam) |
|---|---|---|---|---|---|
| Alpidem | p.o. | 50 | 50 | ||
| Diazepam | p.o. | 6 | 10 | 9.8 (11.2) | 4.3 |
| i.v. | 7.5 | 15 | |||
| i.m. | 7.5 | 15 | |||
| Camazepam | p.o. | 10 | 10 | ||
| Adinazolam | p.o. | 20 | 20 | ||
| Chlorazepate | p.o. | 15 | 15 | ||
| Clobazam | p.o. | 20 | 20 | ||
| Flutoprazepam | p.o. | 2 | 2 | (12.0) | |
| Lorazepam | p.o. | 1 | 1 | 3.8 (2.6) | 1.7 |
| i.v. | 2 | 2 | |||
| Medazepam | p.o. | 15 | 15 | (2322) | |
| Oxazepam | p.o. | 30 | 30 | 39 (37.4) | |
| Premazepam | p.o. | 25 | 25 | ||
| Abecarnil | p.o. | 10 | 10 | ||
| Alprazolam | p.o. | 0.75 | 0.75 | 10.6 (13.8) | 0.6 |
| i.v. | 1 | 1 | |||
| Midazolam | p.o. | 10 | 15 | 4.86 | 2.5 |
| i.v. | 7.5 | 15 | |||
| Quazepam | p.o. | 15 | 15 | 66 | 10.2 |
| Temazepam | p.o. | 10 | 20 | 58 | 10.8 |
| i.v. | 10 | 20 | |||
| Bretazanil | p.o. | 0.5 | 0.5 | ||
| Bromazepam | p.o. | 4.5 | 4.5 | (39.8) | 5.5 |
| i.v. | 4.5 | 6 | |||
| Flunitrazepam | i.v. | 0.5 | 1 | 6.2 |
i.m., intramuscular; i.v., intravenous; p.o., per os.
Table 3.
Tests or parameters that never showed any significant effect after administration of a benzodiazepine registered or investigated for anxiety.
| Test | Reference | Test | Reference |
|---|---|---|---|
| Antisaccadic peak velocity | [46] | Mentally slow/quickwitted | [30] |
| Antisaccadic velocity | [63] | Most/least nauseated | [29] |
| Anxiety | [25, 26, 27, 56] | Normal/easily telded | [60] |
| Attentive/dreamy | [30] | Peaceful/tense | [60] |
| Basle mood scale | [44] | Performance | [44] |
| Blood pressure | [25] | Prepulse inhibition | [27] |
| Bodily symptom scale | [33] | Prolactine | [32] |
| Calm/anxious | [60] | Puff duration | [26] |
| Clearheaded/muzzy | [30] | Pulse rate | [25] |
| Clyde mood scale | [24] | Pursuit aiming | [21] |
| Compensatory effect | [21] | Repeated acquisition (2nd order) | [26] |
| Contendedness | [27, 33, 34, 60, 64] | Restless/calm | [29] |
| Cortical excitability | [38] | Self-rated concentration ability | [65] |
| Differential reinforcement of low response rate | [26] | Sequence completion | [30] |
| Divided visual attention | [16] | Serum gastrin concentrations | [37] |
| Drug liking | [26] | Sternberg memory test | [42] |
| Fatigue | [21] | Stroop colour word test | [21] |
| Functional reach | [11] | Subjective drug potency scale | [26] |
| Gastric acid secretion | [37] | Subjektieve Leistungseinschätzung | [58] |
| Happy/sad | [29] | Subjektieve Stimmung | [58] |
| High | [26] | Tension | [39] |
| Hopkins symptom checklist | [41] | Tone discrimination | [16] |
| HVA | [40] | Two flash fusion | [55] |
| Incompetent/capable | [30] | VAS Mood Scale (no Bond & Lader) | [41] |
| Inter-puff/interval | [26] | Visual attention | [16] |
| Logical reasoning | [56] | Visual search | [56] |
| Long-term visual memory | [16] | Well coordinated/clumsy | [30] |
| Maddox wing | [67] | Wiener Gerät | [44, 53] |
| Max force | [25] | Worst/best ever | [29] |
Neuropsychological/motor skill tests
There were 73 different tests (–variants) as shown in Table 1. Seventeen of these were used only once and 55 tests were used fewer than five times in combination with a benzodiazepine dose. Sixteen tests never showed any significant effects at all. Tests that showed a consistent response across different benzodiazepines include the digit symbol substitution task (DSST), which was measured 33 times and showed significant impairment in 21 of these cases. Tracking showed impairment in eight out of nine cases and visual reaction times showed impairment in three out of five cases. Similarly, the choice reaction time showed impairment in 53% of the 15 observations. The critical flicker fusion was used 16 times and showed impairment in six cases but all these cases include high benzodiazepine dose. Both DSST and tracking showed significant responses at therapeutic doses. The only observation of effects on visual reaction time at a therapeutic dose was not significant. Choice reaction times results were similar at low, medium or high dose; impairment was observed in half the cases. The responsiveness of both DSST and tracking improved after discarding the low dose results.
Subsequently, comparable tests were clustered. The clusters ‘complex information processing’ (9 out of 21), ‘DSST-like’ (25 out of 38), ‘flicker discrimination’ (6 out of 20), ‘hand-eye coordination’ (17 out of 34), ‘manipulation’ (4 out of 11), ‘other vigilance’ (8 out of 17), and ‘reaction time’ (19 out of 34) showed consistent responses across studies. However, at therapeutic doses, only ‘DSST-like’ and ‘hand-eye coordination’ showed responses in half the cases or more. ‘DSST-like’ tests showed the clearest dose–response-relationship (25% significant results for low dose, 67% for medium and 94% for high dose).
Attempts were made to construct a reference dose–response curve for the ‘DSST-like’ cluster.
There were too many different parameters to allow clear dose–response-relationships for any of the 11 benzodiazepines that were studied with ‘DSST-like’ tests. Some studies measured ‘number of correct substituted symbols over 90 s’. Others measured ‘time needed to substitute 90 symbols’ or ‘power of DSST (correct number divided by time needed for correct substitutions)’. the most commonly reported parameter ‘score/90 s’) was plotted against the fraction of therapeutic dose for all benzodiazepines in Figure 1. No clear relationship was observed between this ‘normalized’ therapeutic dose used and the result on the DSST.
Figure 1.
The effects on DSST (r2 = 0.03) and subjective alertness (r2 = 0.29) of benzodiazepine doses normalized to fraction of therapeutic dose.
In order to investigate the overall effects of benzodiazepines on the major neurocognitive domains, the clusters were further condensed to domains. The results are displayed in Figure 2. The results for low, medium and high doses are represented in the same figure. This differentiation showed that most domains are affected by high-dose benzodiazepines.
Figure 2.
The averaged significant effects of benzodiazepines on neuropsychological domains, subjective assessment and neurophysiological parameters (see text for explanation). Averaged overall scores (♦) and effects after low dose ( ), therapeutic (medium) dose (
), therapeutic (medium) dose ( ) and above therapeutic (high) benzodiazepine dose (
) and above therapeutic (high) benzodiazepine dose ( ).
).
Subjective assessments
Fifty-eight different subject assessments were used. Thirty tests never showed any significant effects. Most tests were used fewer than five times (48 assessments) and 15 tests were only used once. The most consistently responsive scale was ‘alertness’, which was significantly impaired in 11 out of 14 cases. Other responsive scales included ‘sedation’ both scored by the subjects and by the investigator (11 out of 14 and three out of five times significant results, respectively). However, the scale ‘sedation’ showed improvement in three cases and impairment in eight cases. Similarly, the investigator-rated ‘sedation’ scale showed improvement once and impairment thrice.
Clustering the results for subjective assessments showed that the scales ‘alertness’, ‘mood’ and ‘calmness’ as described by Norris [68] and adapted by Bond & Lader [69] were frequently employed. The most consistently responding scale was ‘alertness’, which showed 35 reductions and 4 improvements out of the 94 times it was used.
Alertness also complied with the second and third requirement. All observations at medium doses were significant reductions. A quantitative analysis was performed to assess the fourth requirement as shown in Figure 1. This analysis showed no relationship between a decrease in alertness and the different dosages (‘normalized’ for therapeutic dose) of benzodiazepines assessed by alertness. There were too few results to perform a more quantitative analysis of the test alertness.
Neurophysiological assessments
Sixty-two different neurophysiological parameters were identified. Twelve parameters never showed any significant effect and 22 parameters were used only once. Thirty-seven parameters were used fewer than five times.
Electroencephalogram (EEG)
Inconsistent responses were observed for EEG theta: two increases, three decreases and one nonsignificant result. EEG Delta was increased in two cases whereas remained unaffected in three cases. EEG alpha showed significant reductions in five out of eight cases and EEG beta was increased in all five instances.
Eye movements
Eye movement tests were the most consistently responsive tests. Smooth pursuit eye movement recordings (measured 12 times) showed impairment in 50%. Non-/anti-saccadic eye movements were used 13 times and showed impairment in 54%. Saccadic latency showed impairment in 4 out of 9 observations. Saccadic eye movements showed impairment in 80% of all cases and was measured most frequently (31 times). The most frequently used parameter was saccadic peak velocity (11 times) and it showed significant impairment compared to placebo in all cases.
Saccadic peak velocity (SPV) also showed consistent effects at therapeutic doses. A reference dose–response curve could be constructed for temazepam, since saccadic peak velocity was reported in all studies where saccadic eye movements were used with this drug. an Emax model with E0 (placebo response) was used to construct a reference curve using 9 placebo responses and 10 temazepam responses at various doses according to the following equation:
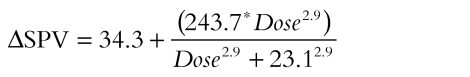 |
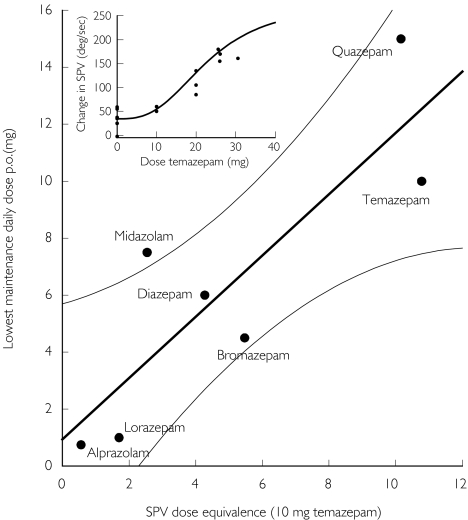
Subsequently, ΔSPV responses of all benzodiazepines were used to calculate the corresponding temazepam dose. These values were averaged for each benzodiazepine and plotted against the lowest recommended therapeutic maintenance dose as shown in Figure 3. a significant correlation was observed for seven benzodiazepines according to the equation (r2 = 0.737, P < 0.05):
Figure 3.
SPV-decreasing dose equivalencies compared with lowest daily therapeutic maintenance dose for various benzodiazepines (see text for explanation). The 95% confidence interval (95% CI) of the linear regression is shown in thin lines. Insert: reference curve for temazepam dose (x-axis) and SPV-decrease relative to baseline (y-axis).
Lowest maintenance dose = 0.94 + 1.08 × SPV dose equivalence.
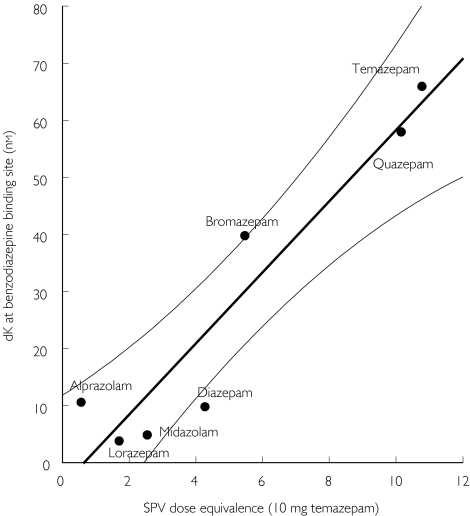
Furthermore, the SPV dose equivalences of these seven benzodiazepines strongly correlated to the Kd at benzodiazepine binding sites (r2 = 0.894, P < 0.01) as shown in Figure 4:
Figure 4.
SPV-decreasing dose equivalencies compared with dissociation constants at benzodiazepine binding site for various benzodiazepines (see text for explanation). The 95% confidence interval (95% CI) of the linear regression is shown in thin lines.
Kd at benzodiazepine binding sites =−4.06 + 6.24 × SPV dose equivalence.
Evoked potentials
Evoked potential tests were used three times and showed impairment in all. Evoked potentials were measured in two studies using two benzodiazepines. Auditory evoked response potentials (AERP) P300 was used once, as was AERP slow wave positivity. These results came from the same study. The auditory 40 Hz response amplitude was used also once in another study.
Startle reflex
Startle reflex tests were used four times and in each case showed benzodiazepine-induced reductions. ‘Startle blink’ was used three times in one study with one benzodiazepine. ‘Acoustic startle’ was used once.
Discussion
The aim of this review was to evaluate the usefulness of methods used in healthy volunteer studies, to assess effects of anxiolytic benzodiazepines. A strikingly large number (173) of different neurocognitive tests were identified. About one-third of all tests used in combination with benzodiazepines [58] never showed any significant response to a benzodiazepine dose. Only very few methods were used often enough to allow individual evaluation. Consequently, tests had to be grouped to observe trends for relationships between comparable tests and benzodiazepine effects. Several different meaningful ways to group tests were used in this review, although each method inevitably led to a loss of information. Even grouping tests with the same name and/or description could bypass differences among research groups or test variants. Some methods only used once or twice or by a single research groups may have had all the characteristics of ideal biomarkers, but this would have been missed in this review, simply because part of the definition of ‘ideal’ was general widespread use of the biomarker. Evoked potentials and startle responses, for instance, showed consistent results, but only in less than a handful of studies from even fewer research groups. At this stage, it is difficult to evaluate the usefulness of these techniques in drug development, and more studies are needed to allow definite judgements. In addition, useful methods were defined in this review as tests that produced a statistically significant result in typical healthy volunteer studies, i.e. with small subject numbers. Some tests may be very useful biomarkers in larger studies, but these would not be identified in this review.
As expected, increasing doses caused more significant results for many tests. The sedative properties of benzodiazepines at high doses caused some impairment in most of the neurocognitive domains, probably secondary to reduced alertness. However, a useful biomarker should show responses at therapeutic levels (preferably also at low doses to allow dose–response relationships). This precludes ‘critical flicker’ discrimination tests, which, despite widespread use, only seem to respond to high dose levels of benzodiazepines. The more useful biomarkers identified in this review (saccadic eye movements, ‘DSST-like’ tests and subjective scores of alertness) all seem to be related to the sedative properties of benzodiazepines, which apparently correlate with the therapeutic effects of the selected benzodiazepines. Effects of other sedating compounds have been demonstrated with saccadic eye movements, suggesting that saccadic eye movements quantitatively reflect alertness [70].
All benzodiazepines caused an impairment of saccadic peak velocity, which was closely related to the therapeutic dose. There are several possible explanations for this close relationship. Firstly, it could reflect the clinical practice of aiming for maximum tolerated levels. Secondly, the anxiolytic effects of benzodiazepines could be linked to sedation ‘in parallel’, if both are regulated by closely related neurobiological systems (e.g. different GABA-receptor subtypes or different components of the ascending reticular activating system; the latter probably connects saccadic eye movements to alertness/sedation). Thirdly and perhaps less plausibly, the link could be ‘in series’, if reduced alertness would be the basis for reducted anxiety (e.g. by reduced susceptibility to (disturbing) exogenous and endogenous stimuli). Research on partial agonist benzodiazepines that potentially discriminate between sedation and anxiolysis should include saccadic peak velocity as the most sensitive measure of sedation. Similarly, the effects of non-benzodiazepine anxiolytic agents could show a different effect profile. A review of the effects of such variable compounds (similar to the current benzodiazepine review) would be difficult, because the diverse effect profiles would hamper any relationship between biomarkers and pharmacology of the drugs. In addition, most of these drugs are registered for multiple indications (e.g. depression).
CNS drug development is likely to increase as the attention of the pharmaceutical industry shifts further in the direction of this area with the largest unmet therapeutic need. Additionally, the improvements in biological knowledge through genomics will undoubtedly produce new targets that require further validation [71]. Early evaluation of these new drugs must be done with the best possible methodology and it is highly surprising that the field apparently uncritically uses untested and often insensitive methodology. Healthy volunteers should not be exposed to procedures that can a priori be assumed not to produce any useful data. In addition, the cost of these studies is high, especially when no or possibly confusing data arise from this. A large number of the methods included in this review are actually used for studies that eventually appear in dossiers for registration and the uncritical approach to this methodology seems to extend to the registration authorities in many countries.
Similar to the conclusion of a review on the effects of antipsychotic drugs in healthy volunteers [1], this review confirms that the number of tests used in human psychopharmacology appears to be excessive, and reduction of the number of tests as well as further evaluation and validation is long overdue.
Acknowledgments
This research was partly funded by R. W. Johnson Pharmaceutical Research Institute, High Wycombe, UK. This research was produced on behalf of the German Association for Applied Human Pharmacology (Arbeitsgemeinschaft für angewandte Humanpharmakologie; AGAH) biomarker working group.
References
- 1.de Visser SJ, Post v.d J, Pieters MSM, Cohen AF, van Gerven JMA. Biomarkers for the effects of antipsychotic drugs in healthy volunteers. Br J Clin Pharmacol. 2001;51:119–132. doi: 10.1111/j.1365-2125.2001.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spreen O, Strauss E. A Compendium of Neuropsychological Tests; Administration, Norms, and Commentary. 2. New York: Oxford University Press, Inc; 1998. (ISBN 0-19-510019-0) [Google Scholar]
- 3.van Steveninck AL, Schoemaker HC, den Hartigh J, et al. Effects of intravenous temazepam. I. Saccadic eye movements and electroencephalogram after fast and slow infusion to pseudo steady state. Clin Pharmacol Ther. 1994;55:535–545. doi: 10.1038/clpt.1994.67. [DOI] [PubMed] [Google Scholar]
- 4.van Steveninck AL, Verver S, Schoemaker HC, et al. Effects of temazepam on saccadic eye movements: concentration-effect relationships in individual volunteers. Clin Pharmacol Ther. 1992;52:402–408. doi: 10.1038/clpt.1992.162. [DOI] [PubMed] [Google Scholar]
- 5.van Steveninck AL, Schoemaker HC, Pieters MS, Kroon R, Breimer DD, Cohen AF. A comparison of the sensitivities of adaptive tracking, eye movement analysis and visual analog lines to the effects of incremental doses of temazepam in healthy volunteers. Clin Pharmacol Ther. 1991;50:172–180. doi: 10.1038/clpt.1991.122. [DOI] [PubMed] [Google Scholar]
- 6.Bodfish JW, Powell SB, Golden RN, Lewis MH. Blink rate as an index of dopamine function in adults with mental retardation and repetitive behavior disorders. Am J Ment Retard. 1995;99:335–344. [PubMed] [Google Scholar]
- 7.Goldberg TE, Maltz A, Bow JN, Karson CN, Leleszi JP. Blink rate abnormalities in autistic and mentally retarded children: relationship to dopaminergic activity. J Am Acad Child Adolesc Psych. 1987;26:336–338. doi: 10.1097/00004583-198705000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Karson CN. Spontaneous eye-blink rates and dopaminergic systems. Brain. 1983;106:643–653. doi: 10.1093/brain/106.3.643. [DOI] [PubMed] [Google Scholar]
- 9.Richelson E, Nelson A, Neeper R. Binding of benzodiazepines and some major metabolites at their sites in normal human frontal cortex in vitro. J Pharmacol Exp Ther. 1991;256:897–901. [PubMed] [Google Scholar]
- 10.Ito K, Asakura A, Yamada Y, Nakamura K, Sawada Y, Iga T. Prediction of the therapeutic dose for benzodiazepine anxiolytics based on receptor occupancy theory. Biopharm Drug Dispos. 1997;18:293–303. doi: 10.1002/(sici)1099-081x(199705)18:4<293::aid-bdd24>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 11.Cutson TM, Gray SL, Hughes MA, Carson SW, Hanlon JT. Effect of a single dose of diazepam on balance measures in older people. J Am Geriatr Soc. 1997;45:435–440. doi: 10.1111/j.1532-5415.1997.tb05167.x. [DOI] [PubMed] [Google Scholar]
- 12.File SE, Fluck E, Joyce EM. Conditions under which lorazepam can facilitate retrieval. J Clin Psychopharmacol. 1999;19:349–353. doi: 10.1097/00004714-199908000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Sambrooks JE, MacCulloch MJ, Rooney JF. The automated assessment of the effect of flurazepam and nitrazepam on mood state. Acta Psychiatr Scand. 1975;51:201–209. doi: 10.1111/j.1600-0447.1975.tb00005.x. [DOI] [PubMed] [Google Scholar]
- 14.Ingum J, Bjorklund R, Volden R, Morland J. Development of acute tolerance after oral doses of diazepam and flunitrazepam. Psychopharmacology (Berl) 1994;113:304–310. doi: 10.1007/BF02245201. [DOI] [PubMed] [Google Scholar]
- 15.Schachinger H, Muller BU, Strobel W, Langewitz W, Ritz R. Midazolam effects on prepulse inhibition of the acoustic blink reflex. Br J Clin Pharmacol. 1999;47:421–426. doi: 10.1046/j.1365-2125.1999.00896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satzger W, Engel RR, Ferguson E, Kapfhammer H, Eich FX, Hippius H. Effects of single doses of alpidem, lorazepam, and placebo on memory and attention in healthy young and elderly volunteers. Pharmacopsychiatry. 1990;23(Suppl 3):114–119. doi: 10.1055/s-2007-1014546. [DOI] [PubMed] [Google Scholar]
- 17.Nielsen-Kudsk F, Jensen TS, Magnussen I, et al. Pharmacokinetics and bioavailability of intravenous and intramuscular lorazepam with an adjunct test of the inattention effect in humans. Acta Pharmacol Toxicol (Copenh) 1983;52:121–127. doi: 10.1111/j.1600-0773.1983.tb03413.x. [DOI] [PubMed] [Google Scholar]
- 18.Tallone G, Ghirardi P, Bianchi MC, Ravaccia F, Bruni G, Loreti P. Reaction time to acoustic or visual stimuli after administration of camazepam and diazepam in man. Arzneimittelforschung. 1980;30:1021–1024. [PubMed] [Google Scholar]
- 19.Unrug A, Coenen A, van Luijtelaar G. Effects of the tranquillizer diazepam and the stimulant methylphenidate on alertness and memory. Neuropsychobiology. 1997;36:42–48. doi: 10.1159/000119359. [DOI] [PubMed] [Google Scholar]
- 20.Kroboth PD, Folan MM, Lush RM, et al. Coadministration of nefazodone and benzodiazepines. I. Pharmacodynamic assessment. J Clin Psychopharmacol. 1995;15:306–319. doi: 10.1097/00004714-199510000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Kozena L, Frantik E, Horvath M. Vigilance impairment after a single dose of benzodiazepines. Psychopharmacology (Berl) 1995;119:39–45. doi: 10.1007/BF02246052. [DOI] [PubMed] [Google Scholar]
- 22.Unrug A, van Luijtelaar EL, Coles MG, Coenen AM. Event-related potentials in a passive and active auditory condition: effects of diazepam and buspirone on slow wave positivity. Biol Psychol. 1997;46:101–111. doi: 10.1016/s0301-0511(96)05237-4. [DOI] [PubMed] [Google Scholar]
- 23.Duka T, Schutt B, Krause W, Dorow R, McDonald S, Fichte K. Human studies on abecarnil a new beta-carboline anxiolytic: safety, tolerability and preliminary pharmacological profile. Br J Clin Pharmacol. 1993;35:386–394. doi: 10.1111/j.1365-2125.1993.tb04155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Christensen P, Lolk A, Gram LF, Kragh-Sorensen P. Benzodiazepine-induced sedation and cortisol suppression. A placebo-controlled comparison of oxazepam and nitrazepam in healthy male volunteers. Psychopharmacology (Berl) 1992;106:511–516. doi: 10.1007/BF02244823. [DOI] [PubMed] [Google Scholar]
- 25.Schaffler K, Klausnitzer W. Single dose study on buspirone versus diazepam in volunteers. Monitoring psychomotor performance via oculomotor, choice reaction and electromyographic parameters. Arzneimittelforschung. 1988;38:282–287. [PubMed] [Google Scholar]
- 26.Kelly TH, Foltin RW, Serpick E, Fischman MW. Behavioral effects of alprazolam in humans. Behav Pharmacol. 1997;8:47–57. [PubMed] [Google Scholar]
- 27.Abduljawad KA, Langley RW, Bradshaw CM, Szabadi E. Effects of clonidine and diazepam on the acoustic startle response and on its inhibition by ‘prepulses’ in man. J Psychopharmacol. 1997;11:29–34. doi: 10.1177/026988119701100110. [DOI] [PubMed] [Google Scholar]
- 28.Yasui N, Otani K, Kaneko S, et al. A kinetic and dynamic study of oral alprazolam with and without erythromycin in humans: in vivo evidence for the involvement of CYP3A4 in alprazolam metabolism. Clin Pharmacol Ther. 1996;59:514–519. doi: 10.1016/S0009-9236(96)90179-4. [DOI] [PubMed] [Google Scholar]
- 29.Risby ED, Hsiao JK, Golden RN, Potter WZ. Intravenous alprazolam challenge in normal subjects. Biochemical, cardiovascular, and behavioral effects. Psychopharmacology (Berl) 1989;99:508–514. doi: 10.1007/BF00589900. [DOI] [PubMed] [Google Scholar]
- 30.Ghoneim MM, Mewaldt SP, Hinrichs JV. Dose–response analysis of the behavioral effects of diazepam. II. Psychomotor performance, cognition and mood. Psychopharmacology (Berl) 1984;82:296–300. doi: 10.1007/BF00427673. [DOI] [PubMed] [Google Scholar]
- 31.Inanaga K, Tanaka M, Mizuki Y. Prediction of clinical efficacy of zopiclone by utilizing two psychophysiological tools in healthy volunteers. Int Pharmacopsych. 1982;17(Suppl 2):109–115. [PubMed] [Google Scholar]
- 32.Noderer J, Duka T, Dorow R. [Benzodiazepine antagonism by RO 15–1788: psychometric, hormonal and biophysical parameters] Anaesthetist. 1988;37:535–542. [PubMed] [Google Scholar]
- 33.Golombok S, Lader M. The psychopharmacological effects of premazepam, diazepam and placebo in healthy human subjects. Br J Clin Pharmacol. 1984;18:127–133. doi: 10.1111/j.1365-2125.1984.tb02444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Neill WM, Hanks GW, White L, Simpson P, Wesnes K. The cognitive and psychomotor effects of opioid analgesics. I. A randomized controlled trial of single doses of dextropropoxyphene, lorazepam and placebo in healthy subjects. Eur J Clin Pharmacol. 1995;48:447–453. doi: 10.1007/BF00194333. [DOI] [PubMed] [Google Scholar]
- 35.Schaffler K, Klausnitzer W. Placebo-controlled study on acute and subchronic effects of buspirone vs bromazepam utilizing psychomotor and cognitive assessments in healthy volunteers. Pharmacopsychiatry. 1989;22:26–33. doi: 10.1055/s-2007-1014573. [DOI] [PubMed] [Google Scholar]
- 36.Healey M, Pickens R, Meisch R, McKenna T. Effects of clorazepate, diazepam, lorazepam, and placebo on human memory. J Clin Psych. 1983;44:436–439. [PubMed] [Google Scholar]
- 37.Stacher G, Bauer P, Brunner H, Grunberger J. Gastric acid secretion, serum-gastrin levels and psychomotor function under the influence of placebo, insulin-hypoglycemia, and/or bromazepam. Int J Clin Pharmacol Biopharm. 1976;13:1–10. [PubMed] [Google Scholar]
- 38.Palmieri MG, Iani C, Scalise A, et al. The effect of benzodiazepines and flumazenil on motor cortical excitability in the human brain. Brain Res. 1999;815:192–199. doi: 10.1016/s0006-8993(98)01164-0. [DOI] [PubMed] [Google Scholar]
- 39.van Steveninck AL, Wallnofer AE, Schoemaker RC, et al. A study of the effects of long-term use on individual sensitivity to temazepam and lorazepam in a clinical population. Br J Clin Pharmacol. 1997;44:267–275. doi: 10.1046/j.1365-2125.1997.t01-1-00580.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingerson DK, Cowley DS, Kramer GL, Petty F, Roy-Byrne PP. Effect of benzodiazepines on plasma levels of homovanillic acid in anxious patients and control subjects. Psych Res. 1996;65:53–59. doi: 10.1016/0165-1781(96)02886-7. [DOI] [PubMed] [Google Scholar]
- 41.Linnoila M, Stapleton JM, Lister R, et al. Effects of single doses of alprazolam and diazepam, alone and in combination with ethanol, on psychomotor and cognitive performance and on autonomic nervous system reactivity in healthy volunteers. Eur J Clin Pharmacol. 1990;39:21–28. doi: 10.1007/BF02657051. [DOI] [PubMed] [Google Scholar]
- 42.Blom MW, Bartel PR, de Sommers K, Van der Meyden CH, Becker PJ. The effects of alprazolam, quazepam and diazepam on saccadic eye movements, parameters of psychomotor function and the EEG. Fundam Clin Pharmacol. 1990;4:653–661. doi: 10.1111/j.1472-8206.1990.tb00045.x. [DOI] [PubMed] [Google Scholar]
- 43.Ochs HR, Greenblatt DJ, Luttkenhorst M, Verburg-Ochs B. Single and multiple dose kinetics of clobazam, and clinical effects during multiple dosage. Eur J Clin Pharmacol. 1984;26:499–503. doi: 10.1007/BF00542148. [DOI] [PubMed] [Google Scholar]
- 44.Hobi V, Dubach UC, Skreta M, Forgo J, Riggenbach H. The effect of bromazepam on psychomotor activity and subjective mood. J Int Med Res. 1981;9:89–97. doi: 10.1177/030006058100900201. [DOI] [PubMed] [Google Scholar]
- 45.Giersch A, Lorenceau J. Effects of a benzodiazepine, lorazepam, on motion integration and segmentation: an effect on the processing of line-ends? Vision Res. 1999;39:2017–2025. doi: 10.1016/s0042-6989(98)00311-3. [DOI] [PubMed] [Google Scholar]
- 46.Green JF, King DJ. The effects of chlorpromazine and lorazepam on abnormal antisaccade and no-saccade distractibility. Biol Psych. 1998;44:709–715. doi: 10.1016/s0006-3223(97)00452-6. [DOI] [PubMed] [Google Scholar]
- 47.Samara EE, Granneman RG, Witt GF, Cavanaugh JH. Effect of valproate on the pharmacokinetics and pharmacodynamics of lorazepam. J Clin Pharmacol. 1997;37:442–450. doi: 10.1002/j.1552-4604.1997.tb04322.x. [DOI] [PubMed] [Google Scholar]
- 48.Stewart SH, Rioux GF, Connolly JF, Dunphy SC, Teehan MD. Effects of oxazepam and lorazepam on implicit and explicit memory: evidence for possible influences of time course. Psychopharmacology (Berl) 1996;128:139–149. doi: 10.1007/s002130050119. [DOI] [PubMed] [Google Scholar]
- 49.Vidailhet P, Kazes M, Danion JM, Kauffmann-Muller F, Grange D. Effects of lorazepam and diazepam on conscious and automatic memory processes. Psychopharmacology (Berl) 1996;127:63–72. doi: 10.1007/BF02805976. [DOI] [PubMed] [Google Scholar]
- 50.Suzuki M, Uchiumi M, Murasaki M. A comparative study of the psychological effects of DN-2327, a partial benzodiazepine agonist, and alprazolam. Psychopharmacology (Berl) 1995;121:442–450. doi: 10.1007/BF02246492. [DOI] [PubMed] [Google Scholar]
- 51.Fafrowicz M, Unrug A, Marek T, van Luijtelaar G, Noworol C, Coenen A. Effects of diazepam and buspirone on reaction time of saccadic eye movements. Neuropsychobiology. 1995;32:156–160. doi: 10.1159/000119316. [DOI] [PubMed] [Google Scholar]
- 52.Suttle AB, Songer SS, Dukes GE, et al. Ranitidine does not alter adinazolam pharmacokinetics or pharmacodynamics. J Clin Psychopharmacol. 1992;12:282–287. [PubMed] [Google Scholar]
- 53.Moser L, Macciocchi A, Plum H, Buckmann Effect of flutoprazepam on skills essential for driving motor vehicles. Arzneimittelforschung. 1990;40:533–535. [PubMed] [Google Scholar]
- 54.Nikaido AM, Ellinwood EHJ, Heatherly DG, Gupta SK. Age-related increase in CNS sensitivity to benzodiazepines as assessed by task difficulty. Psychopharmacology (Berl) 1990;100:90–97. doi: 10.1007/BF02245796. [DOI] [PubMed] [Google Scholar]
- 55.Currie D, Lewis RV, McDevitt DG, Nicholson AN, Wright NA. Central effects of beta-adrenoceptor antagonists. I – Performance and subjective assessments of mood. Br J Clin Pharmacol. 1988;26:121–128. doi: 10.1111/j.1365-2125.1988.tb03378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Herbert M, Standen PJ, Short AH, Birmingham AT. A comparison of some psychological and physiological effects exerted by zetidoline (DL308) and by oxazepam. Psychopharmacology (Berl) 1983;81:335–339. doi: 10.1007/BF00427573. [DOI] [PubMed] [Google Scholar]
- 57.Bittencourt PR, Wade P, Smith AT, Richens A. Benzodiazepines impair smooth pursuit eye movements. Br J Clin Pharmacol. 1983;15:259–262. doi: 10.1111/j.1365-2125.1983.tb01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hobi V, Kielholz P, Dubach UC. [The effect of bromazepam on fitness to drive (author's transl)] MMW Munch Med Wochenschr. 1981;123:1585–1588. [PubMed] [Google Scholar]
- 59.Ogle CW, Turner P, Markomihelakis H. The effects of high doses of oxprenolol and of propranolol on pursuit rotor performance, reaction time and critical flicker frequency. Psychopharmacologia. 1976;46:295–299. doi: 10.1007/BF00421117. [DOI] [PubMed] [Google Scholar]
- 60.Kaplan GB, Greenblatt DJ, Ehrenberg BL, Goddard JE, Harmatz JS, Shader RI. Differences in pharmacodynamics but not pharmacokinetics between subjects with panic disorder and healthy subjects after treatment with a single dose of alprazolam. J Clin Psychopharmacol. 2000;20:338–346. doi: 10.1097/00004714-200006000-00008. [DOI] [PubMed] [Google Scholar]
- 61.Blin O, Jacquet A, Callamand S, et al. Pharmacokinetic-pharmacodynamic analysis of mnesic effects of lorazepam in healthy volunteers. Br J Clin Pharmacol. 1999;48:510–512. doi: 10.1046/j.1365-2125.1999.00047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hassan PC, Sproule BA, Naranjo CA, Herrmann N. Dose–response evaluation of the interaction between sertraline and alprazolam in vivo. J Clin Psychopharmacol. 2000;20:150–158. doi: 10.1097/00004714-200004000-00006. [DOI] [PubMed] [Google Scholar]
- 63.Green JF, King DJ, Trimble KM. Antisaccade and smooth pursuit eye movements in healthy subjects receiving sertraline and lorazepam. J Psychopharmacol. 2000;14:30–36. doi: 10.1177/026988110001400103. [DOI] [PubMed] [Google Scholar]
- 64.O'Neill WM, Hanks GW, Simpson P, Fallon MT, Jenkins E, Wesnes K. The cognitive and psychomotor effects of morphine in healthy subjects: a randomized controlled trial of repeated (four) oral doses of dextropropoxyphene, morphine, lorazepam and placebo. Pain. 2000;85:209–215. doi: 10.1016/s0304-3959(99)00274-2. [DOI] [PubMed] [Google Scholar]
- 65.van Steveninck AL, Gieschke R, Schoemaker HC, et al. Pharmacodynamic interactions of diazepam and intravenous alcohol at pseudo steady state. Psychopharmacology (Berl) 1993;110:471–478. doi: 10.1007/BF02244655. [DOI] [PubMed] [Google Scholar]
- 66.van Steveninck AL, Gieschke R, Schoemaker RC, et al. Pharmacokinetic and pharmacodynamic interactions of bretazenil and diazepam with alcohol. Br J Clin Pharmacol. 1996;41:565–573. doi: 10.1046/j.1365-2125.1996.38514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aranko K, Mattila MJ, Seppala T. Development of tolerance and cross-tolerance to the psychomotor actions of lorazepam and diazepam in man. Br J Clin Pharmacol. 1983;15:545–552. doi: 10.1111/j.1365-2125.1983.tb02088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Norris H. The action of sedatives on brain stem oculomotor systems in man. Neuropharmacology. 1971;10:181–191. doi: 10.1016/0028-3908(71)90039-6. [DOI] [PubMed] [Google Scholar]
- 69.Bond A, Lader M. Self-concepts in anxiety states. Br J Med Psychol. 1976;49:275–279. doi: 10.1111/j.2044-8341.1976.tb02375.x. [DOI] [PubMed] [Google Scholar]
- 70.Visser S, Jd Gerven JM, Av Schoemaker HC, Cohen AF. Concentration-effect relationships of two infusion rates of the imidazoline antihypertensive agent rilmenidine for blood pressure and development of side-effects in healthy subjects. Br J Clin Pharmacol. 2001;51:423–428. doi: 10.1046/j.1365-2125.2001.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lehman Brothers. The Fruits of Genomics, 2001. Lehman Brothers Equity Research; 2001. [Google Scholar]