Abstract
Aims
The cytochrome P450 enzyme CYP2C9 catalyses the 4′-hydroxylation of the nonsteroidal analgesic drug diclofenac in humans. We studied the influences of the known amino acid variants, CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu), on diclofenac pharmacokinetics after a 50-mg oral dose of diclofenac in healthy volunteers. As a surrogate marker of diclofenac activity, the ex vivo formation of prostaglandin E2 and thromboxane B2, which reflects COX-2 and COX-1 activity, was measured.
Methods
Genotyping was performed in 516 healthy volunteers to obtain 20 participants with all allelic combinations of the two CYP2C9 variants Arg144Cys (*2) and Ile359Leu (*3). Diclofenac and 4′-hydroxydiclofenac were quantified in plasma by reversed phase h.p.l.c. after oral intake of 50 mg diclofenac. Concentrations of thromboxane B2 (TxB2) and prostaglandin E2 (PGE2) were measured by immunoassays.
Results
There was no evidence of impaired metabolism of oral diclofenac in heterozygous and homozygous carriers of the CYP2C9 alleles *2 and *3 compared with the wild type (mean CL/F (95% CI) 20.5 (11, 30) l h−1 for *1/*1, 29.9 (19, 40) l h−1 for *1/*2, 30.0 (4, 56) l h−1 for *2/*2, 22.6 (12, 33) l h−1 for *1/*3, 23.5 (11, 37) l h−1 for *3/*3 and 37.3 (−15, 89) l h−1 in *2/*3). Furthermore, plasma concentrations of the metabolite 4′-hydroxydiclofenac were not lower in carriers of the CYP2C9 low-activity alleles *2 and *3 compared with carriers of the CYP2C9*1/*1 genotype. Marked diclofenac mediated inhibition of COX-1- and COX-2 activity was detected in all individuals independent of CYP2C9 genotype.
Conclusions
Polymorphisms of the CYP2C9 gene had no discernible effect on the pharmacokinetics and pharmacodynamics of diclofenac. The question of whether enzymes other than CYP2C9 play a major role in diclofenac 4′-hydroxylation in vivo or whether 4′-hydroxylation is not a rate-limiting step in diclofenac elimination in vivo, or whether the effect of the CYP2C9 polymorphisms is substrate-dependent, needs further investigation.
Keywords: COX-1, COX-2, cytochrome P450, CYP2C9, diclofenac, NSAID, prostaglandin E2, thromboxane B2
Introduction
Diclofenac is a widely used nonsteroidal anti-inflammatory drug (NSAID), which acts by potent inhibition of both cyclooxygenase isoenzymes, COX-1 and COX-2. It is approved for the long-term treatment of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis, and also for the short-term treatment of acute musculoskeletal injury, postoperative pain and dysmenorrhoea [1]. Diclofenac produces adverse drug reactions in about 20% of patients. These are mostly gastrointestinal effects, but depression of renal function and elevation of hepatic aminotransferases can also occur [2]. Diclofenac is predominantly eliminated via hepatic biotransformation with less than 1% of the dose being excreted unchanged via the kidneys. The major primary metabolite of diclofenac is 4′-hydroxydiclofenac (4′-OH diclofenac), with 3′-OH- and 5′-OH-diclofenac being minor metabolites [3, 4]. Both diclofenac and its hydroxylated metabolites undergo glucuronidation and sulphation.
In vitro studies with human hepatocytes, liver microsomes and transgenically expressed cytochrome P450 enzymes indicated that cytochrome P450 (CYP) 2C9 almost exclusively catalyses the 4′-hydroxylation of diclofenac [5–8] as well as hydroxylation to the minor metabolite 3′-OH-diclofenac [8]. However, hydroxylation at the 5′-position appeared to be catalysed predominantly by CYP3A4 and to a lesser extent by CYP2C19, CYP2C8 and CYP2C18 [5, 6, 8, 9]. 5′-Hydroxylation may be clinically relevant; Bort et al. suggest that this reaction may be implicated in the hepatotoxicity of diclofenac [10].
CYP2C9 is a genetically polymorphic enzyme that is involved in the biotransformation of many drugs such as phenytoin, losartan, and torasemide, vitamin K antagonists such as S-warfarin and acenocoumarol, oral antidiabetic drugs such as tolbutamide, glipizide, glibenclamide and nateglinide, and NSAIDs such as ibuprofen, naproxen, celecoxib and diclofenac [11–22].
Three alleles of CYP2C9 are relatively frequent in Caucasian populations and exhibit different activities. CYP2C9*1 codes for the wild-type enzyme. In allele CYP2C9*2, arginine144 is changed to cysteine (Cys144-Ile359) and in allele CYP2C9*3, isoleucine359 is changed to leucine (Arg144-Leu359). The fourth possible haplotype from these two single nucleotide polymorphisms, namely Cys144 and Leu359, has never been found in humans thus far. According to in vitro data and human pharmacokinetic studies, the activity of the enzyme coded by CYP2C9*2 is only moderately decreased compared with that of the wild type [23], whereas activity of the CYP2C9*3 gene product is between 5- and 10-fold less depending on the substrate studied [11, 15, 18, 24]. The population frequencies in Caucasians are about 82% for CYP2C9*1, 11% for CYP2C9*2 and 7% for CYP2C9*3[25]. Several other CYP2C9 alleles have been described, which have very low population frequencies in Caucasians (e.g. CYP2C9*6, the only known allele to produce a product with no enzyme activity [26]) or might even be cloning artefacts [24]. Recently, seven variants located in the 5′-flanking region of the CYP2C9 gene were reported, which appear to influence in vivo CYP2C9 activity [27].
The 4′-hydroxylation of diclofenac is reported to be mediated exclusively by CYP2C9 [6] and is the major metabolic pathway at lower substrate concentrations (10 µm) in vitro[5]. Km values for 4′-hydroxylation by CYP2C9 (3.9–22 µm[7, 28–31]); are in the range of peak plasma concentrations following normal doses of 50–100 mg (1.4–17 µmCmax[32]). Thus, genetic polymorphisms in CYP2C9 may influence diclofenac pharmacokinetics, efficacy and adverse events.
In the present study, we wanted to evaluate the influence of the CYP2C9 amino acid variants on diclofenac pharmacokinetics in humans. In addition, we measured inhibition of cyclooxygenases 1 and 2 (COX-1 and COX-2) by diclofenac in healthy volunteers.
Methods
Subjects
From 516 genotyped healthy volunteers, 20 males with all possible combinations of the CYP2C9 alleles *1, *2 and *3 were asked to participate in the study. Demographic data and the CYP2C9, CYP2D6 and CYP2C19 genotypes of the study participants are given in Table 1. Genotyping of CYP2D6 and CYP2C19 was also performed in order to exclude a systematic effect of other polymorphic CYP enzymes. The sample size was chosen as a minimum to be able to detect clinically relevant two-fold or higher differences between two groups with a power of 90% and type I error of 5% based on an earlier published mean AUC of 4.4 mg l−1 h with a standard deviation of 0.8 mg l−1 h in healthy volunteers taking 100 mg of an enteric-coated diclofenac [33]. All participants were nonsmokers and abstained from caffeine- or alcohol-containing beverages as well as from grapefruit foodstuffs during the course of the study. The pre-study health check consisted of a physical examination, laboratory tests, including blood cell counts and hepatic function tests, urine analysis and an electrocardiogram. All volunteers gave written informed consent. The study protocol was approved by the Ethics committee of the Charité university medical centre of the Humboldt University of Berlin.
Table 1.
Demographic data and CYP2C9, CYP2D6 and CYP2C19 genotypes from the study participants.
| Code | Age(years) | Height(cm) | Body weight(kg) | CYP2C9 genotype | CYP2D6 genotype | CYP2C19 genotype |
|---|---|---|---|---|---|---|
| 1 | 26 | 198 | 82 | *1/*1 | *1/*4 | *1/*1 |
| 16 | 25 | 192 | 85 | *1/*1 | *1/*4 | *1/*1 |
| 25 | 25 | 174 | 82 | *1/*1 | *4/*5 | *1/*1 |
| 2 | 27 | 195 | 85 | *1/*2 | *1/*1 | *1/*1 |
| 22 | 31 | 178 | 83 | *1/*2 | *1/*1 | *1/*1 |
| 23 | 30 | 178 | 73 | *1/*2 | *1/*1 | *1/*1 |
| 24 | 39 | 175 | 65 | *1/*2 | *1/*5 | *1/*2 |
| 7 | 24 | 192 | 82 | *1/*3 | *1/*1 | *1/*1 |
| 11 | 31 | 185 | 85 | *1/*3 | *1/*1 | *1/*1 |
| 12 | 24 | 185 | 67 | *1/*3 | *1/*4 | *1/*1 |
| 20 | 57 | 173 | 64 | *1/*3 | *1/*4 | *1/*1 |
| 3 | 36 | 178 | 63 | *2/*2 | *1/*3 | *1/*1 |
| 18 | 29 | 171 | 72 | *2/*2 | *4/*4 | *1/*1 |
| 33 | 30 | 174 | 81 | *2/*2 | *1/*6 | *1/*1 |
| 4 | 24 | 180 | 67 | *2/*3 | *1/*4 | *1/*1 |
| 9 | 23 | 185 | 75 | *2/*3 | *1/*4 | *1/*1 |
| 19 | 30 | 192 | 72 | *2/*3 | *1/*1 | *1/*1 |
| 5 | 45 | 178 | 79 | *3/*3 | *1/*1 | *1/*1 |
| 13 | 29 | 176 | 71 | *3/*3 | *1/*6 | *1/*1 |
| 14 | 26 | 179 | 74 | *3/*3 | *1/*1 | *1/*1 |
| Mean | 31 | 182 | 75 | |||
| SD | 8 | 8 | 8 |
After a fasting period of 12 h, 50 mg diclofenac sodium was administered orally in its enteric-coated form (Voltaren®, Novartis Pharma) and plasma samples were taken at 0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, 10, 24, 28, 34 and 48 h. Four hours after drug intake, a standard lunch was served. The intake of tap water was allowed throughout the study.
Genotyping procedure
DNA was extracted from 5 ml EDTA blood samples using a standard phenol-chloroform extraction method. DNA samples were dissolved in 10 mm Tris/1 mm EDTA, pH 8.0 and stored at 4 °C. PCR-RFLP was used to detect the different alleles of CYP2C9 as described earlier [34]. PCR for analysis of CYP2C9*2 resulted in a 372-bp fragment which was digested by Sau96I to 179-, 119- and 74-bp fragments in the presence of the wild-type allele and to 253- and 119-bp fragments in the case of CYP2C9*2. PCR for the analysis of CYP2C9*3 resulted in a 130-bp amplicon, which was cut into 104- and 26-bp fragments by digestion with StyI, whereas the wild-type remained uncut.
Analyses for the CYP2D6 alleles *3, *4, *5, *6 and the duplication as well as for the CYP2C19*2 were performed using methods described earlier [35, 36]. To validate CYP2C9 genotyping, all analyses were performed twice. No discrepant results were found.
Analysis of diclofenac plasma concentrations
Diclofenac and racemic flurbiprofen (internal standard) were from Sigma (Deisenhofen, Germany) and 4′-OH diclofenac was from Gentest Inc. (Woburn, Massachusetts, USA). All solvents and reagents were h.p.l.c. grade (Merck, Darmstadt, Germany). After thawing, the plasma samples were centrifuged to remove possible precipitates and 500 µl samples were transferred to an extraction vial, mixed with 20 µl of a methanolic solution of 100 ng of the internal standard and 200 µl 1 m phosphoric acid. Extraction into 4 ml n-hexane/diethylether (50 : 50, v/v) was performed twice. The combined extracts were evaporated under nitrogen and dissolved in 150 µl acetonitrile/water (50 : 50, v/v) for injection onto the h.p.l.c. system.
Chromatography was performed on a LiChrospher 100RP18™ (Merck, Darmstadt, Germany) reversed phase column (column dimensions: 4 mm × 125 mm) at a flow rate of 1.5 ml min−1 with u.v. detection at 280 nm, using gradient elution. Eluent A was prepared from 0.02 m phosphate buffer, pH 3.0 and acetonitrile (95 : 5, v/v), and eluent B was prepared with the same phosphate buffer and acetonitrile (45 : 55, v/v). A linear gradient starting from 25% eluent B to 100% eluent B over 15 min was used for elution. Retention times were 7.8 min (4′-OH diclofenac), 14.1 min (IS) and 14.9 min (diclofenac). The coefficient of variation for 4′-OH diclofenac was 12.8% at 200 µg l−1 and 15.6% at 800 µg l−1 level. In all samples from three of the participants of this study, analysis for glucuronide and sulphate metabolites of diclofenac and 4′-OH diclofenac was performed as described after incubation of 0.5 ml plasma samples for 1 h with 5000 units glucuronidase/arylsulphatase (type H-5, Sigma) at 37 °C in sodium acetate buffer, pH 4. The hydrolysis steps did not increase diclofenac plasma concentrations.
The lower limit of quantification (LOQ) defined as half the concentration of the lowest calibrator (50 µg l−1), was 25 µg l−1 for diclofenac and 4′-OH diclofenac. Intraday-variability at 50 µg l−1 was 5.6% for diclofenac and 5.2% for 4′-OH (n = 25). Inter-assay variability for diclofenac was 14.8% at 200 µg l−1 and 8.0% at 800 µg l−1.
Thromboxane B2 (TxB2) and prostaglandin E2 (PGE2) analyses
Whole blood samples without anticoagulant were drawn at the same pre-dose times as the diclofenac samples during the first 10 h of the study. To measure NSAID mediated inhibition of TxB2 generation (presumed to be generated by constitutively expressed platelet COX-1), each sample was incubated for 1 h at 37 °C prior to separation of plasma by centrifugation. Plasma was kept at −70 °C until assayed for TxB2. To measure diclofenac mediated inhibition of the formation of PGE2 (presumed to be generated by inducible COX-2), heparinized blood, drawn at the same times, was treated with 10 µg ml−1Escherichia coli lipopolysaccharide serotype 026:B6 (Sigma) and was incubated for 24 h at 37 °C. Plasma was separated by centrifugation and kept at −70 °C until assayed for PGE2.
Thromboxane and prostaglandin were quantified using a commercial thromboxane B2 and prostaglandin E2 enzyme immunoassays from Biotrend (Cologne, Germany) by methods described elsewhere [37–39]. The measurement was performed by using an automatic plate reader (Spectra II, SLT Labinstruments) with the instrument control and data analysis software Magellan® (Spectra II, SLT, Tecan Crailsheim, Germany). The samples were diluted with assay buffer according to the assay instructions to obtain concentrations in the defined calibration range. Intra-assay coefficients of variation for thromboxane were between 1.6 and 4.0% for concentrations between 760 and 2600 ng l−1, and interassay coefficients of variation ranged from 3.6% to 7.6% for concentrations between 44 and 3000 ng l−1. The limit of determination was 7.98 ng l−1.
For prostaglandin E2, intra-assay coefficients of variation ranged from 5.8 to 17.5% over a concentration range of 116–2416 ng l−1. Inter-assay coefficients of variation ranged from 3.0 to 5.1% between concentrations of 111–1902 ng l−1. The limit of determination was 36 ng l−1.
Pharmacokinetic analysis
For each patient, 16 plasma samples for concentration analysis were drawn, but in most patients, plasma concentrations were below the quantification limit during the lag-time of about 1.5 h and at 8 h after intake. Oral clearances and AUCs(0,8 h) were described by a noncompartmental analysis using WINNONLIN version 1.5, 1997 (Scientific Consulting Inc., NC, USA). Oral clearances were calculated as dose/AUC with extrapolation to infinity. The three last data points of the elimination phase were used to determine the elimination rate constant.
Statistics
For testing statistical significance of differences between the CYP2C9 genotypes, the Jonckheere–Terpstra trend test was used to test for gene–dose-dependent tends, as implemented in the SPSS software (SPSS for Windows, version 10; SPSS Inc., Chicago, IL, USA). The a priori defined trend for the CYP2C9 genotypes was in the following order: CYP2C9*1/*1, *1/*2, *2/*2, *1/*3, *2/*3, and *3/*3.
Sample size estimation and post hoc power analysis were performed using the program Nquery version 4 (Statistical Solutions Inc., Cork, Ireland) based on comparison of two groups using Student's t-test. The most important comparison was that between individuals not possessing CYP2C9*3 alleles (*1/*1,*1/*2 and*2/*2) and individuals possessing one or more CYP2C9*3 alleles (*1/*3,*2/*3 and*3/*3). This is because CYP2C9*3 showed the largest functional effect in studies with other CYP2C9 substrates whereas CYP2C9*2 expression seems to alter CYP2C9 enzyme activity only slightly.
Results
All participants tolerated the study medication well and completed the study. The pharmacokinetic parameters of diclofenac and 4′-OH diclofenac are shown in Table 2. The oral clearance (CL/F) of diclofenac varied about four-fold, with a mean value of 27.2 l h−1 (95% confidence interval [CI] of the mean: 21.5, 33.1 l h−1). After a mean lag-time of 01.22 h (0.78, 1.66 h), the mean time to reach maximal plasma concentrations was 01.98 h (1.5, 2.4 h). There was more than 10-fold variability in maximal diclofenac plasma concentrations (Cmax) with a mean of 1.7 mg l−1 (1.4, 2.1 mg l−1). The decline in plasma-concentrations followed a mono-exponential function with a mean half-life of 0.9 h (0.75, 01.05 h).
Table 2.
Pharmacokinetic data of diclofenac in the 20 male study participants classified by CYP2C9 genotype.
| Diclofenac | 4′-OH Diclofenac | |||||||
|---|---|---|---|---|---|---|---|---|
| CYP2C9 genotype | Code | Clearance/F(L h−1) | tlag(h) | Cmax(µg L−1) | t1/2(h) | AUC last(µg h L−1) | tmax(h) | Cmax(µg L−1) |
| *1/*1 | 1 | 17.1 | 1.08 | 3124 | 0.89 | 321 | 2.33 | 96 |
| 16 | 19.2 | 0.55 | 1797 | 1.8 | – | – | – | |
| 25 | 24.4 | 1.62 | 1473 | 0.77 | 423 | 3.1 | 122 | |
| Mean | 20.2 | 1.08 | 2131 | 1.2 | 372 | 2.7 | 109 | |
| *1/*2 | 2 | 23.6 | 1.00 | 2331 | 0.42 | 126 | 2.50 | 94 |
| 22 | 32.5 | 0.53 | 910 | 1.3 | 402 | 2.03 | 100 | |
| 23 | 24.2 | 0.58 | 1719 | 1.2 | 34 | 2.53 | 29 | |
| 24 | 34.8 | 1.2 | 1034 | 1.2 | 266 | 3.17 | 89 | |
| Mean | 28.8 | 0.83 | 1498 | 1.0 | 207 | 2.56 | 78 | |
| *1/*3 | 7 | 31.3 | 3.18 | 1151 | 0.65 | 102 | 5.17 | 80 |
| 11 | 16.7 | 0.58 | 2462 | 1.4 | 502 | 2.08 | 102 | |
| 12 | 22.7 | 1.03 | 1498 | 0.98 | 86 | 2.57 | 39 | |
| 20 | 18.8 | 0.02 | 2604 | 1.2 | 210 | 1.53 | 142 | |
| Mean | 22.4 | 1.20 | 1929 | 1.1 | 225 | 2.84 | 91 | |
| *2/*2 | 3 | 26.7 | 1.07 | 1554 | 1.1 | 380 | 2.10 | 101 |
| 18 | 21.3 | 1.52 | 2963 | 1.1 | 342 | 2.52 | 116 | |
| 33 | 41.5 | 0.58 | 1405 | 0.27 | 221 | 2.08 | 205 | |
| Mean | 29.8 | 1.06 | 1974 | 0.81 | 314 | 2.23 | 141 | |
| *2/*3 | 4 | 71.5 | 1.55 | 278 | 1.5 | 281 | 6.05 | 98 |
| 9 | 32.6 | 4.07 | 734 | 0.85 | 813 | 5.03 | 540 | |
| 19 | 18.5 | 1.02 | 2229 | 0.91 | 267 | 2.02 | 125 | |
| Mean | 40.9 | 2.21 | 1081 | 1.1 | 453 | 4.37 | 254 | |
| *3/*3 | 5 | 25.7 | 1.05 | 1418 | 0.97 | 306 | 2.57 | 119 |
| 5 | 25.7 | 1.05 | 1418 | 0.97 | 306 | 2.57 | 119 | |
| 13 | 17.1 | 0.6 | 1650 | 1.1 | 841 | 2.10 | 99 | |
| 14 | 26.5 | 1.57 | 1764 | 1.3 | 181 | 2.58 | 58 | |
| Mean | 23.1 | 1.07 | 1610 | 1.1 | 442 | 2.42 | 92 | |
| All | Mean (95%CI) | 27.3 (21.5–33.1) | 1.2 (0.8–1.7) | 1704 (1360–2050 | 1.0 (0.88–1.2) | 321 (217–425) | 2.8 (2.3–3.4) | 124 (72–176) |
**4′-OH diclofenac could not be analysed in this subject.
CYP2C9 genotype
In Figure 1, the individual oral clearances are shown divided into groups according to the six allelic combinations of the CYP2C9 gene. Neither CYP2C9*2 nor CYP2C9*3 showed an influence on pharmacokinetic parameters of diclofenac. Low clearances were even found in the homozygous carriers of the wild-type allele. Assuming equal variances, the 95% CI on the differences between the mean oral clearances of subject with and without a CYP2C9*3 allele was −13.5 and 10.3, which spans a large nearly symmetrical range around 0. The power of our study to detect differences of at least 50% in oral clearances in these groups of n = 10 was determined as 70%.
Figure 1.
The effect of the CYP2C9 genotype on the oral clearance of diclofenac in healthy subjects.
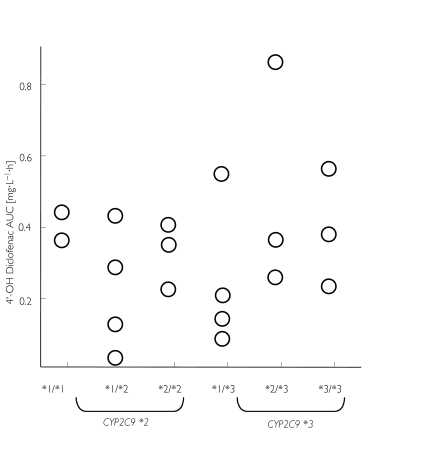
Correspondingly, no influence of CYP2C9 genotype on the plasma concentrations of 4′-OH diclofenac was detected. The individual areas under the curve (AUC(0, 12 h)) of 4′-OH diclofenac in the different genotype groups are shown in Figure 2. In Figure 3, the plasma concentration-time courses from subjects with the homozygous CYP2C9 genotypes *1/*1, *2/*2 and *3/*3 are depicted. Carriers of the less active alleles *2 and *3 of CYP2C9 did not show higher diclofenac concentrations than carriers of two wild-type alleles *1.
Figure 2.
The effect of the CYP2C9 genotype on the AUC0−12 h of 4′-OH diclofenac.
Figure 3.
The effect of the CYP2C9 genotype on the plasma concentration-time curves of (a) diclofenac, (b) thromboxane B2 and (c) prostaglandin E2. From left, CYP2C9 *1/1; CYP2C9 *2/*2; CYP2C9 *3/*3. Data from CYP2C9 heterozygotes are not illustrated.
Serum thromboxane B2 (TxB2)
A large decline in TxB2 concentrations to values below 5 µg l−1 was seen in all volunteers about 1–2 h after dosing, and TxB2 concentrations remained 50% below the baseline for 2.3 h in mean. The TxB2 concentration-time courses from representative subjects with homozygous genotype *1/*1, *2/*2 and *3/*3 are depicted in Figure 3. The baseline values, the periods of time, when inhibition was more than 50%, and AUC(0,10 h) values are given in Table 3. In all subjects, inhibition of more than 90% was observed. No differences were seen between the CYP2C9 genotypes.
Table 3.
Parameters of thromboxane B2 and prostaglandin E2 and indicator of COX-1-/COX-2-activity in the subjects classified by CYP2C9 genotype.
| TxB2 | PgE2 | ||||||
|---|---|---|---|---|---|---|---|
| CYP2C9 genotype | Code | Baselineconcentration(µg L−1) | AUC0−10(µg/L h) | period of 50%inhibitio(h) | Baselineconcentration(µg L−1) | AUC0−10(µg/L h) | period of conc. < 10 µg L−1(h) |
| *1/*1 | 1 | 175 | 1164 | 3.5 | 92 | 378 | 1.7 |
| 16 | 140 | 1386 | 1.5 | 61 | 269 | 3.7 | |
| 25 | 50 | 385 | 3.8 | 21 | 81 | 6.2 | |
| Mean | 122 | 978 | 2.9 | 58 | 242 | 3.9 | |
| *1/*2 | 2 | 130 | 1111 | 1.8 | 55 | 338 | 3.4 |
| 22 | 75 | 544 | 2.5 | 18 | 35 | 9.2 | |
| 23 | 70 | 1325 | 1.7 | 247 | 303 | 3.5 | |
| 24 | 100 | 1672 | 1.3 | 200 | 490 | 2.4 | |
| Mean | 94 | 1163 | 1.8 | 130 | 292 | 4.6 | |
| *1/*3 | 7 | 110 | 578 | 1.9 | 76 | 443 | 5.2 |
| 11 | 75 | 516 | 2.9 | 33 | 215 | 3.3 | |
| 12 | 105 | 639 | 3.2 | 30 | 220 | 3.9 | |
| 20 | 50 | 740 | 1.8 | 23 | 78 | 4.7 | |
| Mean | 85 | 618 | 2.5 | 41 | 239 | 4.3 | |
| *2/*2 | 3 | 30 | 240 | 1.4 | 54 | 238 | 2.4 |
| 18 | 120 | 1257 | 2.3 | 249 | 460 | 3.0 | |
| 33 | 145 | 2488 | 1.5 | 107 | 258 | 4.7 | |
| Mean | 98 | 1329 | 1.7 | 137 | 318 | 3.4 | |
| *2/*3 | 4 | 120 | 1479 | 2.3 | 47 | 90 | 5.5 |
| 9 | 100 | 479 | 3.0 | 114 | 876 | 1.4 | |
| 19 | 120 | 1172 | 3.7 | 14 | 51 | 10.2 | |
| Mean | 113 | 1043 | 3.0 | 59 | 339 | 5.7 | |
| *3/*3 | 5 | 45 | 430 | 1.6 | 102 | 346 | 3.7 |
| 13 | 80 | 826 | 2.5 | 63 | 201 | 3.4 | |
| 14 | 150 | 1535 | 2.0 | 102 | 319 | 4.3 | |
| Mean | 92 | 930 | 2.0 | 89 | 288 | 3.8 | |
| All | Mean(95%CI) | 100(81–118) | 949(701–1196) | 2.3(1.9–2.7) | 85(52–119) | 284(193–376) | 4.3(3.3–5.3) |
Baseline: drawn from values before decline; AUC 0–10: Area under the concentration-time course during the first 10 h after medication; TxB2 period of 50% inhibition: period of time with TxB2 values under 50% of baseline; PgE2 period of conc. < 10 µg L−1: period of time with PgE2-concentrations smaller than 10 µg L−1.
Plasma prostaglandin E2 (PGE2)
A steep decline in plasma PGE2 concentrations was seen about 1 h after diclofenac intake, which had not yet fully recovered 12 h post-dose. Concentration-time courses of PGE2 are shown in Figure 3 for the three homozygous genotypic groups. PGE2 concentrations at baseline (before medication) varied more than 15-fold. After diclofenac medication, PGE2 values remained below 10 µg l−1 for a mean of 4.3 h. PGE2 inhibition was detected throughout the decline in diclofenac concentrations. Individual data for the baseline PGE2 concentrations, the periods of time with PGE2 concentrations below 10 µg l−1 and the AUC(0,10 h) are given in Table 3. Concentrations of PGE2 did not depend on CYP2C9 genotypes.
Discussion
The present study aimed to evaluate the effect of CYP2C9 genetic polymorphisms on the pharmacokinetics of diclofenac in healthy volunteers. Groups representing each combination of three CYP2C9 alleles were included. Based on in vitro data we anticipated that individuals homozygous for the allele type *3/*3 of CYP2C9 who are phenotypically slow metabolizers of other CYP2C9 substrates such as tolbutamide [15] would have a greatly reduced oral clearance of diclofenac, and that heterozygotes would have intermediately reduced clearances compared with carriers of the wild-type genotype *1/*1. However, we failed to observe any difference in the oral clearance of carriers of CYP2C9 alleles *2 and *3 compared with carriers of the wild-type genotype.
A formal post hoc power analysis showed that variability in our group was higher than anticipated from the AUC data of the literature [33]. In our study, the mean of AUCs was 2066 µg l−1 h calculated for all study participants and s.d. was 604 µg l−1 h. For detection of a 100% higher AUC with a power 90% and a type 1 error of 5%, three subjects would be sufficient. In addition, this power analysis is based on two-group comparisons and the power of our study is increased by the joint evaluation of six different groups which should reveal a significant trend if the tested CYP2C9 polymorphisms played a relevant role in diclofenac clearance. A genetic effect on oral clearance should be detectable as a trend of decreasing clearance, with wild type having the highest clearance, heterozygous having intermediate clearance and homozygous carriers of the variant alleles should have the lowest clearance. However, no such trends were observed and carriers of one *3 allele (genotype groups *1/*3 and *2/*3) had on average even higher clearances then the wild-type group (Figure 1).
These data are in good agreement with other recently completed clinical trials. The study of Shimamoto et al. also failed to detect a difference in the diclofenac total clearance between six heterozygous carriers of the CYP2C9*1/*3 genotype compared with homozygous wildtypes [40] using an oral dose of 50 mg diclofenac (Voltaren, Novartis Pharma, Osaka, Japan). Our study complemented these data by including also the ‘extreme’ groups of homozygous carriers of *2/*2, *2/*3 and particularly, *3/*3. Furthermore, another study did not find significant differences between carriers of the CYP2C9*1/*1 genotype vs heterozygous carriers of alleles *2 and *3[41], although these authors found that the mean total clearance of diclofenac in individuals with the genotype CYP2C9*1/*3 (n = 4) was only about 60% and in heterozygous for CYP2C9*1/*2 (n = 3) about 80% of the clearance in subjects with the wild-type genotype CYP2C9*1/*1. Another study from Dorado et al. described a slight, but not statistically significant, elevation of the metabolic ratio diclofenac/4′-OH diclofenac in heterozygous carriers of the alleles *3 and *2 compared with that in homozygous wild-type subjects [42]. These data were based on the comparison of the heterozygous carriers of CYP2C9*2 and *3 with the homozygous wild-type allele combination. Although a codominant mode of inheritance has been demonstrated for cytochrome P450 enzymes such as CYP2C9, the power of studies based on heterozygotes may be lower than those involving CYP2C9*2/*2 and *3/*3. Therefore, we particularly emphasize the comparison of homozygous wildtype subjects with homozygous carriers CYP2C9*2 and *3 (Figure 3). The absence of an effect of the CYP2C9*2 or CYP2C9*3 alleles on diclofenac pharmacokinetics confirms the work of Yasar et al., who also did not find any influence of the CYP2C9 alleles *2 and *3 in one homozygous carrier and four heterozygous carriers after ingestion of a single dose of diclofenac [43].
The pharmacokinetic data were supplemented by measurements of established surrogate markers reflecting the activity of NSAIDs in human tissues. These surrogate markers did not reveal any effect of the CYP2C9 polymorphisms, as would be suspected from the pharmacokinetic data. Such surrogate markers provide important additional information as any pharmacologically active metabolite would also be reflected in the prostanoid concentrations. As expected, in all study participants a marked decline of serum TxB2 and plasma PGE2 was observed, indicating inhibition of COX-1 and COX-2. In some subjects, TxB2 concentrations measured following recovery from inhibition of COX were even higher than the concentrations before diclofenac intake (see Figure 3). It cannot be determined from our study design whether this is due to a circadian rhythm of thromboxane synthesis [44] or to a disputed rebound phenomenon [45, 46]. Comparison of the recovery of TxB2 and PGE2 concentrations indicated that diclofenac might be a more potent inhibitor of PGE2 synthesis. This may be interpreted as a preferential inhibition of COX-2 by diclofenac, which has been described [47]. However, extensive tissue binding of diclofenac within the lymphocytes may also contribute to the prolonged suppression of PGE2 synthesis.
A number of in vitro studies have indicated that CYP2C9 catalyses almost exclusively the formation of 4′-OH diclofenac [5–8]. The Km of this reaction is low and of the same order of magnitude as diclofenac blood concentrations after therapeutic dosages.
The apparent discrepancy between these in vitro data and the results of the clinical studies could be due to several reasons. CYP2C9 may not be the clinically relevant diclofenac hydroxylase in humans; 4′-hydroxylation may not be rate-limiting for oral clearance of diclofenac; or the effect of the CYP2C9 amino acid variants, particularly the leucine 359 variant, may be substrate-dependent.
As we did not quantify 4′-OH diclofenac in the urine, we cannot determine the effect of CYP2C9 genotype on metabolic clearance by 4′-hydroxylation. However, as the recovery of 4′-OH diclofenac in urine and bile accounted for only about 40% of the dose [3], a genetic deficiency in this pathway may not affect overall drug clearance very much.
The amino-acid substitution of the CYP2C9*3 enzyme is located near a known substrate recognition site [48]. In vitro data indicate a differential effect of the CYP2C9 Leu359 variant on Km and Vmax values for different substrates [28, 30, 31, 49].
In conclusion, we found that polymorphisms of the CYP2C9 gene had no discernible effect on the pharmacokinetics and pharmacodynamics of diclofenac. The resolution of the discrepancies between in vitro and in vivo data on the role of CYP2C9 in diclofenac metabolism needs further investigation.
Acknowledgments
The very skilful technical assistance of Mrs Ines Müller is gratefully acknowledged. This work was supported by the German Ministry of Education and Research, BMBF Grant no. 01 GG 9845/5 and by the Berlin Center for Genome Based Bioinformatics, grant 031U209B.
References
- 1.Brogden RN, Heel RC, Pakes GE, Speight TM, Avery GS. Diclofenac sodium: a review of its pharmacological properties and therapeutic use in rheumatic diseases and pain of varying origin. Drugs. 1980;20:24–48. doi: 10.2165/00003495-198020010-00002. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien WM. Adverse reactions to nonsteroidal anti-inflammatory drugs. Diclofenac compared with other nonsteroidal anti-inflammatory drugs. Am J Med. 1986;80:70–80. doi: 10.1016/0002-9343(86)90084-7. [DOI] [PubMed] [Google Scholar]
- 3.Stierlin H, Faigle JW. Biotransformation of diclofenac sodium (Voltaren) in animals and in man. II. Quantitative determination of the unchanged drug and principal phenolic metabolites, in urine and bile. Xenobiotica. 1979;9:611–621. doi: 10.3109/00498257909042328. [DOI] [PubMed] [Google Scholar]
- 4.Stierlin H, Faigle JW, Sallmann A, et al. Biotransformation of diclofenac sodium (Voltaren) in animals and in man. I. Isolation and identification of principal metabolites. Xenobiotica. 1979;9:601–610. doi: 10.3109/00498257909042327. [DOI] [PubMed] [Google Scholar]
- 5.Tang W, Stearns RA, Wang RW, Chiu SH, Baillie TA. Roles of human hepatic cytochrome P450s 2C9 and 3A4 in the metabolic activation of diclofenac. Chem Res Toxicol. 1999;12:192–199. doi: 10.1021/tx9802217. [DOI] [PubMed] [Google Scholar]
- 6.Mancy A, Antignac M, Minoletti C, et al. Diclofenac and its derivatives as tools for studying human cytochromones P450 active sites: Particular efficiency and regioselectivity of P450 2Cs. Biochemistry. 1999;38:14264–14270. doi: 10.1021/bi991195u. [DOI] [PubMed] [Google Scholar]
- 7.Leemann T, Transon C, Dayer P. Cytochrome P450TB (CYP2C): a major monooxygenase catalyzing diclofenac 4′-hydroxylation in human liver. Life Sci. 1993;52:29–34. doi: 10.1016/0024-3205(93)90285-b. [DOI] [PubMed] [Google Scholar]
- 8.Bort R, Mace K, Boobis A, et al. Hepatic metabolism of diclofenac: role of human CYP in the minor oxidative pathways. Biochem Pharmacol. 1999;58:787–796. doi: 10.1016/s0006-2952(99)00167-7. [DOI] [PubMed] [Google Scholar]
- 9.Shen S, Marchick MR, Davis MR, Doss GA, Pohl LR. Metabolic activation of diclofenac by human cytochrome P450 3A4: role of 5-hydroxydiclofenac. Chem Res Toxicol. 1999;12:214–222. doi: 10.1021/tx9802365. [DOI] [PubMed] [Google Scholar]
- 10.Bort R, Ponsoda X, Jover R, Gomez-Lechon MJ, Castell JV. Diclofenac toxicity to hepatocytes: a role for drug metabolism in cell toxicity. J Pharmacol Exp Ther. 1999;288:65–72. [PubMed] [Google Scholar]
- 11.Kidd RS, Straughn AB, Meyer MC, et al. Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics. 1999;9:71–80. doi: 10.1097/00008571-199902000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Odani A, Hashimoto Y, Otsuki Y, et al. Genetic polymorphism of the CYP2C subfamily and its effect on the pharmacokinetics of phenytoin in Japanese patients with epilepsy. Clin Pharmacol Ther. 1997;62:287–292. doi: 10.1016/S0009-9236(97)90031-X. [DOI] [PubMed] [Google Scholar]
- 13.Veronese ME, Mackenzie PI, Doecke CJ, et al. Tolbutamide and phenytoin hydroxylations by cDNA-expressed human liver cytochrome P4502C9. Biochem Biophys Res Commun. 1991;175:1112–1118. doi: 10.1016/0006-291x(91)91680-b. [DOI] [PubMed] [Google Scholar]
- 14.Stearns RA, Chakravarty PK, Chen R, Chiu SH. Biotransformation of losartan to its active carboxylic acid metabolite in human liver microsomes. Role of cytochrome P4502C and 3A subfamily members. Drug Metab Dispos. 1995;23:207–215. [PubMed] [Google Scholar]
- 15.Sullivan-Klose TH, Ghanayem BI, Bell DA, et al. The role of the CYP2C9-Leu359 allelic variant in the tolbutamide polymorphism. Pharmacogenetics. 1996;6:341–349. doi: 10.1097/00008571-199608000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Miners JO, Rees DL, Valente L, Veronese ME, Birkett DJ. Human hepatic cytochrome P450 2C9 catalyzes the rate-limiting pathway of torsemide metabolism. J Pharmacol Exp Ther. 1995;272:1076–1081. [PubMed] [Google Scholar]
- 17.Rettie AE, Korzekwa KR, Kunze KL, et al. Hydroxylation of warfarin by human cDNA-expressed cytochrome P-450: a role for P-4502C9 in the etiology of (S)-warfarin–drug interactions. Chem Res Toxicol. 1992;5:54–59. doi: 10.1021/tx00025a009. [DOI] [PubMed] [Google Scholar]
- 18.Steward DJ, Haining RL, Henne KR, et al. Genetic association between sensitivity to warfarin and expression of CYP2C9*3. Pharmacogenetics. 1997;7:361–367. doi: 10.1097/00008571-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava PK, Yun CH, Beaune PH, Ged C, Guengerich FP. Separation of human liver microsomal tolbutamide hydroxylase and (S) -mephenytoin 4′-hydroxylase cytochrome P-450 enzymes. Mol Pharmacol. 1991;40:69–79. [PubMed] [Google Scholar]
- 20.Davies NM, McLachlan AJ, Day RO, Williams KM. Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet. 2000;38:225–242. doi: 10.2165/00003088-200038030-00003. [DOI] [PubMed] [Google Scholar]
- 21.Klose TS, Ibeanu GC, Ghanayem BI, et al. Identification of residues 286 and 289 as critical for conferring substrate specificity of human CYP2C9 for diclofenac and ibuprofen. Arch Biochem Biophys. 1998;357:240–248. doi: 10.1006/abbi.1998.0826. [DOI] [PubMed] [Google Scholar]
- 22.Yasar U, Tybring G, Hidestrand M, et al. Role of CYP2C9 polymorphism in losartan oxidation. Drug Metab Dispos. 2001;29:1051–1056. [PubMed] [Google Scholar]
- 23.Furuya H, Fernandez Salguero P, Gregory W, et al. Genetic polymorphism of CYP2C9 and its effect on warfarin maintenance dose requirement in patients undergoing anticoagulation therapy. Pharmacogenetics. 1995;5:389–392. doi: 10.1097/00008571-199512000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Stubbins MJ, Harries LW, Smith G, Tarbit MH, Wolf CR. Genetic analysis of the human cytochrome P450 CYP2C9 locus. Pharmacogenetics. 1996;6:429–439. doi: 10.1097/00008571-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Yasar U, Eliasson E, Dahl ML, et al. Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun. 1999;254:628–631. doi: 10.1006/bbrc.1998.9992. [DOI] [PubMed] [Google Scholar]
- 26.Kidd RS, Curry TB, Gallagher S, et al. Identification of a null allele of CYP2C9 in an African-American exhibiting toxicity to phenytoin. Pharmacogenetics. 2001;11:803–808. doi: 10.1097/00008571-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Shintani M, Ieiri I, Inoue K, et al. Genetic polymorphisms and functional characterization of the 5′-flanking region of the human CYP2C9 gene: in vitro and in vivo studies. Clin Pharmacol Ther. 2001;70:175–182. doi: 10.1067/mcp.2001.117367. [DOI] [PubMed] [Google Scholar]
- 28.Takanashi K, Tainaka H, Kobayashi K, et al. CYP2C9 Ile359 and Leu359 variants: enzyme kinetic study with seven substrates. Pharmacogenetics. 2000;10:95–104. doi: 10.1097/00008571-200003000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Carlile DJ, Hakooz N, Bayliss MK, Houston JB. Microsomal prediction of in vivo clearance of CYP2C9 substrates in humans. Br J Clin Pharmacol. 1999;47:625–635. doi: 10.1046/j.1365-2125.1999.00935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki H, Inoue K, Chiba K, et al. Comparative studies on the catalytic roles of cytochrome P450 2C9 and its Cys- and Leu-variants in the oxidation of warfarin, flurbiprofen, and diclofenac by human liver microsomes. Biochem Pharmacol. 1998;56:243–251. doi: 10.1016/s0006-2952(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi H, Kashima T, Nomoto S, et al. Comparisons between in vitro and in vivo metabolism of (S)-warfarin: catalytic activities of cDNA-expressed CYP2C9, its Leu359 variant and their mixture versus unbound clearance in patients with the corresponding CYP2C9 genotypes. Pharmacogenetics. 1998;8:365–373. doi: 10.1097/00008571-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 32.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 33.Macia MA, Frias J, Carcas AJ, et al. Comparative bioavailability of a dispersible formulation of diclofenac and finding of double plasma peaks. Int J Clin Pharmacol Ther. 1995;33:333–339. [PubMed] [Google Scholar]
- 34.Aynacioglu AS, Brockmöller J, Bauer S, et al. Frequency of cytochrome P450 CYP2C9 variants in a Turkish population and functional relevance for phenytoin. Br J Clin Pharmacol. 1999;48:409–415. doi: 10.1046/j.1365-2125.1999.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sachse C, Brockmöller J, Bauer S, Roots I. Cytochrome P450 2D6 variants in a Caucasian population: allele frequencies and phenotypic consequences. Am J Hum Genet. 1997;60:284–295. [PMC free article] [PubMed] [Google Scholar]
- 36.de Morais SM, Wilkinson GR, Blaisdell J, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J Biol Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 37.Patrignani P, Panara MR, Greco A, et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J Pharmacol Exp Ther. 1994;271:1705–. 12. [PubMed] [Google Scholar]
- 38.Patrono C, Ciabattoni G, Pugliese F, et al. Radioimmunoassay of serum thromboxane B2: a simple method of assessing pharmacologic effects on platelet function. Adv Prostaglandin Thromboxane Res. 1980;6:187–191. [PubMed] [Google Scholar]
- 39.Patrono C, Ciabattoni G, Pugliese F, et al. Radioimmunoassay measurement of stable metabolites of platelet arachidonic acid: a convenient method for the in vitro and ex vivo evaluation of cyclo-oxygenase inhibitors. Agents Actions Suppl. 1980;7:256–259. [PubMed] [Google Scholar]
- 40.Shimamoto J, Ieiri I, Urae A, et al. Lack of differences in diclofenac (a substrate for CYP2C9) pharmacokinetics in healthy volunteers with respect to the single CYP2C9*3 allele. Eur J Clin Pharmacol. 2000;56:65–68. doi: 10.1007/s002280050722. [DOI] [PubMed] [Google Scholar]
- 41.Morin S, Loriot MA, Poirier JM, et al. Is diclofenac a valuable CYP2C9 probe in humans? Eur J Clin Pharmacol. 2001;56:793–797. doi: 10.1007/s002280000240. [DOI] [PubMed] [Google Scholar]
- 42.Dorado R, Norberto M-J, Berecz R, et al. CYP2C9 genotype and diclofenac hydroxylation in a Spanish population. Pharmacol Toxicol. 2001;89:102. [Google Scholar]
- 43.Yasar Ü, Eliasson E, Forslund-Bergengren C, et al. The role of CYP2C9 genotype in the metabolism of diclofenac in vivo and in vitro. Pharmacol Toxicol. 2001;89:106. doi: 10.1007/s00228-001-0376-7. [DOI] [PubMed] [Google Scholar]
- 44.Vacas MI, Del Zar MM, Martinuzzo M, et al. Inhibition of human platelet aggregation and thromboxane B2 production by melatonin. Correlation with plasma melatonin levels. J Pineal Res. 1991;11:135–139. doi: 10.1111/j.1600-079x.1991.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 45.Vial JH, McLeod LJ, Roberts MS. Rebound elevation in urinary thromboxane B2 and 6-keto-PGF1 alpha excretion after aspirin withdrawal. Adv Prostaglandin Thromboxane Leukot Res. 1991;21A:157–160. [PubMed] [Google Scholar]
- 46.Seppala E, Laitinen O, Vapaatalo H. Comparative study on the effects of acetylsalicylic acid, indomethacin and paracetamol on metabolites of arachidonic acid in plasma, serum and urine in man. Int J Clin Pharmacol Res. 1983;3:265–269. [PubMed] [Google Scholar]
- 47.Warner TD, Giuliano F, Vojnovic I, et al. Nonsteroid drug selectivities for cyclo-oxygenase-1 rather than cyclo-oxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gotoh O. Substrate recognition sites in cytochrome P450 family 2 (CYP2) proteins inferred from comparative analyses of amino acid and coding nucleotide sequences. J Biol Chem. 1992;267:83–90. [PubMed] [Google Scholar]
- 49.Aithal GP, Day CP, Leathart JB, Daly AK. Relationship of polymorphism in CYP2C9 to genetic susceptibility to diclofenac-induced hepatitis. Pharmacogenetics. 2000;10:511–518. doi: 10.1097/00008571-200008000-00004. [DOI] [PubMed] [Google Scholar]