Abstract
Aims
To determine the population pharmacokinetics of theophylline during extracorporeal membrane oxygenation (ECMO) from routine monitoring data.
Methods
Retrospective data were collected from 75 term neonates and children (age range 2 days to 17 years) receiving continuous infusions of aminophylline (mean rate 9.2 ± 2.6 µg kg−1 min−1) during ECMO. A total of 160 plasma concentrations (range 1–8 per patient), sampled at time intervals ranging from 10 h to 432 h, were included. Population PK analysis and model building were carried out using WinNonMix Professional (Version 2.0.1). Cross-validation was used to evaluate the validity and predictive accuracy of the model.
Results
A one-compartment model with first order elimination combined with an additive error model was found to best describe the data. Of the covariables tested, bodyweight significantly influenced clearance and volume of distribution, whereas age was an important determinant of clearance, as adjudged by the differences in the −2 × log likelihood (P < 0.005) and the residual error value. The final model parameters were estimated as: clearance (l h−1) = 0.023 × bodyweight (kg) + 0.000057 × age (days) and volume of distribution (l) = 0.57 × bodyweight (kg). The interindividual variability in clearance and volume of distribution was 38% and 40%, respectively. The residual error corresponded to a standard deviation of 3.6 mg l−1. Cross-validation revealed a median (95% confidence interval) model bias of 9.4% (2.9, 16.5%) and precision of 29.5% (24.8, 36.0%).
Conclusions
The estimated clearance is significantly lower, and volume of distribution higher, than previously reported in non-ECMO patients of similar age. These differences are probably a result of the expanded circulating volume during ECMO and altered renal and hepatic physiology in this critically ill group. Large interindividual variability reflects the heterogeneous nature of patients treated on ECMO.
Keywords: extracorporeal membrane oxygenation, population pharmacokinetics, theophylline
Introduction
There is substantial evidence supporting the diuretic and naturetic effects of theophylline both in normal subjects and adult patients with congestive cardiac failure [1, 2]. More recently it has been shown to be beneficial in the management of fluid overload in critically ill patients [3, 4]. The pharmacokinetics of theophylline in paediatric patients have been extensively studied, suggesting that age and weight are the most important determinants of clearance and volume of distribution [5–15]. However, there are no pharmacokinetic studies in patients receiving extracorporeal membrane oxygenation (ECMO).
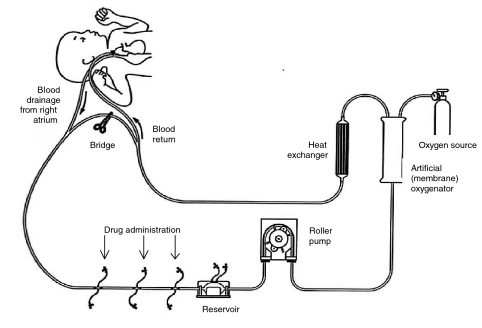
ECMO is a life-support technique used in the management of patients with severe pulmonary or cardiopulmonary failure (Figure 1) [16, 17]. Previous studies have shown the disposition of drugs to be affected during ECMO, probably as a result of the expanded circulating volume and interactions with the polymeric components of the extracorporeal circuit [18–20]. At this institution, continuous infusions of aminophylline are commonly employed during ECMO (in conjunction with conventional agents) for its diuretic properties and in response to renal failure or to manage fluid overload.
Figure 1.
Schematic diagram of a paediatric ECMO circuit.
The purpose of this investigation was therefore to characterize the pharmacokinetics of theophylline in this specialist population and to test the predictive performance and validity of the derived pharmacokinetic model.
A population approach enables the estimation of pharmacokinetic parameters using sparse drug concentration data obtained from a large number of patients and the determination of influence of patient factors (covariables) on these parameters through utilizing routinely available clinical data [21, 22]. This method also allows the estimates of interpatient variability of these parameters and any residual error in the model that may result from intrapatient variability, measurement error and model mis-specification. Such an approach is particularly attractive in the paediatric population, as it does not require a large number of blood samples per patient. Furthermore, as the method employs data generated during routine care, it does not require the patients to be managed under the rigours of a traditional study protocol.
Materials and methods
Data were collected retrospectively from medical records and routine therapeutic monitoring database for theophylline, in 75 children admitted for ECMO on the paediatric intensive care unit. Eligibility criteria included all patients receiving aminophylline during ECMO with at least one serum concentration measurement. The Leicestershire ethics committee approved the study. The ECMO circuit used in the study population consisted of Norton S-65HL polyvinyl chloride tubing (Tygon®; Norton Performance Plastics, Corby, Northants, UK) and a silicone membrane oxygenator (Model 0800; Avecor, Cardiovascular Inc., Minneapolis, MN, USA). The approximate priming volumes of the circuit depend on the patient's age and bodyweight and are displayed in Table 1.
Table 1.
Priming volumes of extracorporeal circuits with respect to other variables.
| Patient weight range (kg) | Oxygenator (AVECOR) | Priming volume (ml) | Normal circulating volumes*(ml) | Approximate increase in circulating volume (%) |
|---|---|---|---|---|
| ≤4.9 | 0800 | 500 | Up to 400 | 125 |
| 5–9.9 | 1500 | 600 | Up to 792 | 75 |
| 10–49 | Ultrox II or | 1200 | Up to 4000 | 25 |
| Ultrox III | 1000 | |||
| ≥50 | 2 × Ultrox I or | 2200 | Up to 5-6000 | 25–33 |
| 2 × Ultrox II | 1500 |
Calculated from an assumed circulating volume of 80 ml kg−1[42].
Theophylline was administered as a continuous intravenous infusion of aminophylline into the ECMO circuit prereservoir, starting at a rate of between 5 and 15 µg kg−1 min−1. Except for two patients, no loading doses were administered. The decision to initiate treatment once the patient had been cannulated for ECMO was made by the attending clinician and based on clinical and biochemical status. All blood samples were taken from an arterial line at time intervals determined by the attending clinician or clinical pharmacist. The precise date and time of sampling were recorded on the analysis request form and transferred with the final results of the analysis onto a computer database. Serum concentrations were analysed by the Olympus Theophylline enzyme immunoassay method (Olympus Diagnostica GmbH, Hamburg, Germany). The limit of determination was 0.8 mg l−1 and the intra-assay coefficients of variation at 5.5, 13.9 and 26.9 mg l−1 were 3.0%, 1.9% and 1.5%, respectively.
Demographic and clinical characteristics documented for each serum sample were: age (postnatal age in neonates), weight, gender, cannulation mode [veno-venous (VV) or venoarterial (VA)], pertinent medical history (congenital heart defect, sepsis/pneumonia, congenital diaphragmatic hernia), urea and creatinine, ECMO flow rates, comedication (macrolide and quinolone antibiotics, imidazole antifungals, phenytoin and phenobarbitone), liver function tests, requirements for renal replacement therapy (continuous veno-venous haemofiltration (CVVH)) and C-reactive protein.
Population pharmacokinetic analysis
Population pharmacokinetic modelling was performed using WinNonMix Professional (Version 2.0.1; Pharsight Corp., Mountain View, CA, USA) bundled with Compaq Visual Fortran compiler (Professional Edition, Version 6.5; Compaq Computer Corp., Houston, TX, USA) [23, 24]. In a preliminary analysis, one- and two-compartment models with first-order elimination were fitted to all data from all subjects simultaneously. The one-compartment model was selected for further analysis, as it demonstrated a more appropriate structural model on examination of the graphical diagnostic plots as well as the differences in the objective function value (OFV), ie two times the negative log likelihood value. Initial parameter estimates were obtained from a previous report of population pharmacokinetics in a paediatric population [12]. Once the base model had been defined, the influence of various demographic and clinical covariables in the regression models for clearance and volume of distribution were evaluated. Each covariate was added sequentially and then (where significant) cumulatively to the initial regression model in a linear and nonlinear manner. In addition, exponential functions of weight were also modelled as reported by previous studies. The influence of this model building process on the weighted residuals and the change in the OFV was noted. The difference in the OFV obtained before and after the addition of covariables is approximately χ2 distributed with degrees of freedom equal to the number of parameters that are set to the null hypothesis value. A change in the OFV > 7.88 (P < 0.005) was accepted as statistically significant.
Interpatient variability
The interpatient variability in clearance and volume of distribution was modelled as a proportional (constant coefficient of variation) deviation from the ‘true’ parameter values (assuming a log normal distribution).
βi = β× ebi
βI = True value of a structural parameter (clearance and volume) in the ith individual
β = Population parameter value predicted by the regression model
bi = Random variable, which is normally distributed with mean zero and represents the interpatient variability in the study population.
Residual error
The residual error (intrapatient variability), which describes the difference between the observed and the predicted concentrations, was also estimated:
Cij = The jth observed concentration in the ith individual
Cij = The jth predicted concentration in the ith individual
ɛij = Residual error, normally distributed with mean zero
The variance for ɛij, σ2, was modelled as:
Fixing a to 1 and b to 0 produces an additive error model, whereas fixing a to 0 and b to 1 produces a proportional error model. Both of these models were also compared to an additive plus proportional error model (a > 0, b = 1). Again, the diagnostic plots and OFV were used to find the most appropriate error model.
Initial modelling was carried out using the first-order linearization method. The final fit of the basic model and subsequent covariate testing were conducted using the first-order conditional estimation method.
Model validation
The predictive performance of our model was tested using the cross-validation technique [25], as it was not practical to collect new data prospectively. Although cross-validation is not a truly prospective validation method, it is a recognized and established approach to estimating model performance assuming identical experimental conditions [26–28].
The study group was randomly divided into five smaller groups of 15 patients each. The pharmacokinetic model was fitted to the data when excluding one group. The estimated structural parameters were then used to predict the concentrations in the excluded group. This process was repeated four times excluding each group in turn. A measure of the predictive performance of models can be determined by calculating the median prediction error (MDPE), ie observed minus predicted concentrations, and the absolute median prediction error (MDAPE) [29]. The MDPE describes the bias and MDAPE the precision (variability) of the predictions. As the excluded group is not used to develop the model, the MDPE and the MDAPE are almost unbiased estimates of the predictive capabilities of the model [27].
Results
Demographics
The clinical and demographic characteristics of the study population are shown in Table 2. A wide range of patients was included, with respect to age, weight and diagnoses. All neonates were term, and accounted for 49% of the total group. Patients were sedated with continuous infusions of a benzodiazepine and opioid combination and received antibiotics based on culture and sensitivity results or as prophylaxis. In 31% of cases, aminophylline was initiated on day 1 of ECMO (range 1–12 days). A mean of 2.2 (range 1–8) serum theophylline concentrations per patient were analysed. Samples were taken over a wide range of times (median 88.4, range 9.8–431.7 h) giving a median concentration of 15 mg l−1 (range 1–38.8). Serum concentration exceeded 20 mg l−1 in 27% of cases. Although 10 patients required CVVH, no patient exhibited significant liver dysfunction other than neonatal hyperbilirubinaemia.
Table 2.
Demographic and clinical characteristics of the study population
| Overall | Neonates(0–1 month) | Infants(1–12 months) | Children(>12 months) | |
|---|---|---|---|---|
| Number of patients | 75 | 38 | 14 | 23 |
| Gender | ||||
| Males | 39 | 27 | 2 | 10 |
| Females | 36 | 17 | 11 | 8 |
| Weight (kg) | 10.3 ± 15.7 | 3.3 ± 0.5 | 4.8 ± 2.0 | 25.3 ± 22.1 |
| (2.1–85) | (2.1–4.1) | (2.7–9.0) | (9.3–85) | |
| Age (days) | 713 ± 1487 | 8.4 ± 5.9 | 122 ± 107 | 2236 ± 1980 |
| (2–6205) | (2–28) | (29–368) | (394–6205) | |
| Cannulation | ||||
| VV | 57 | 32 | 10 | 15 |
| VA | 18 | 12 | 3 | 3 |
| Diagnosis | ||||
| Post cardiothoracic surgery | 12 | 4 | 3 | 5 |
| MAS/PPHN/RDS | 22 | 22 | ||
| CDH | 10 | 9 | 1 | |
| ARDS | 7 | 7 | ||
| Pneumonia | 11 | 2 | 3 | 6 |
| Sepsis | 2 | 1 | 1 | |
| RSV/CLD | 5 | 4 | 1 | |
| Other | 6 | 1 | 3 | 2 |
| CVVH | 10 | 7 | 2 | 1 |
| Comedication | 29 | 10 | 7 | 12 |
| Number of serum concentrations | 160 | 72 | 37 | 51 |
| Number > 20 mg l−1 | 43 | 22 | 8 | 13 |
| Infusion dose (µg kg−1 min−1) | 9.2 ± 2.6 | |||
| (4–16.6) |
Mean ± s.d. (range). VV, Veno-venous; VA, venoarterial; MAS, meconium aspiration syndrome; PPHN, persistent pulmonary hypertension of the newborn; RDS, respiratory distress syndrome; CDH, congenital diaphragmatic hernia; ARDS, acute respiratory distress syndrome; RSV, respiratory syncytial virus; CLD, chronic lung disease; CVVH, continuous veno-venous haemofiltration.
Model development
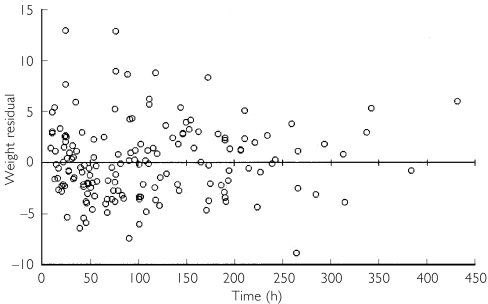
A one-compartment model with first-order elimination, associated with a proportional error model for interpatient variability and an additive error model for residual error, produced the best scatter plots of weighted residuals (Figure 2), and the lowest OFV values. During the covariate screening phase, only weight and age significantly reduced the OFV (>7.88) when added individually. No other covariate significantly contributed to the variability in the structural parameters and consequently resulted in poor parameter estimates.
Figure 2.
Weighted results vs. time. Weighted residuals = residual (observed - predicted) concentration divided by the square root of the observed concentration variance
A significant correlation between clearance and weight (r2 = 0.97) as well as age (r2 = 0.13) was demonstrable. In the cumulative model building process, weight was optimally modelled as a continuous function on clearance and volume. Exponential functions of weight resulted in very similar changes in OFV, and thus the simpler model was chosen. Although the parameter estimate for age in clearance was not estimated very precisely (coefficient of variation (CV) 96.4%), its inclusion did significantly improve the model. In contrast, the combination of age with weight in volume did not show an improvement, and thus age was omitted from volume (Table 3). The parameter estimates of the final structural and error models are shown in Table 4.
Table 3.
Covariate model building: effect on objective function value (OFV) of the significant covariables weight and age as determinants of clearance and volume
| Addition of covariable | (OFV)* | P-value† | Conclusion |
|---|---|---|---|
| CL, V (base model, with no covariate influence) | 1154 | ||
| CL with weight, V (with no covariate influence) | 1061 | <0.005 | Weight significantly influences clearance |
| CL (with no covariate influence), V with weight | 1116 | <0.005 | Weight significantly influences volume |
| CL with weight, V with weight | 995 | <0.005 | |
| CL with weight and age, V with weight | 985 | <0.005 | Age significantly influences clearance |
| CL with weight, V with weight and age | 989 | >0.005 | Age does not significantly influence volume |
−2 × log likelihood.
Assuming χ2 distribution, a change in the OFV > 7.88 (P < 0.005) was accepted as statistically significant.
Table 4.
Population pharmacokinetic parameter details for the final model of theophylline in paediatric ECMO patients
| Estimate | Standard error | CV (%) | |
|---|---|---|---|
| β1 | 0.023 | 0.0016 | 6.9 |
| β2 | 0.000 057 | 0.000 055 | 96.4 |
| β3 | 0.57 | 0.053 | 9.3 |
| Interpatient variability in clearance | 0.15 (38.7%) | 0.038 | 25.3 |
| Interpatient variability in volume | 0.16 (40%) | 0.13 | 81.2 |
| Residual error | 13.0 (s.d. = 3.6 mg l−1) | 3.09 | 23.7 |
CL (l h−1) =β1 × weight (kg) + β2 × age (days). V (l) =β3 × weight (kg).
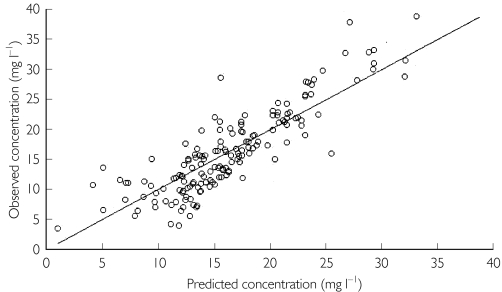
The values of clearance and volume were estimated with high precision, giving (CVs of 6.9% and 9.3%, respectively). The CVs of interpatient variability for clearance and volume were 38.7% and 40% (precision 25.3% and 81.2%, respectively). The additive error model for residual error estimated a standard deviation of 3.6 mg l−1. As this error is constant over the concentrations studied, the CVs in serum theophylline concentrations of 5, 10 and 20 mg l−1 would be 72%, 36% and 18%, respectively. The relationship between observed and final model predictions is shown in Figure 3.
Figure 3.
Observed vs. individual predicted serum theophylline concentrations using the final model.
Predictive performance and cross-validation
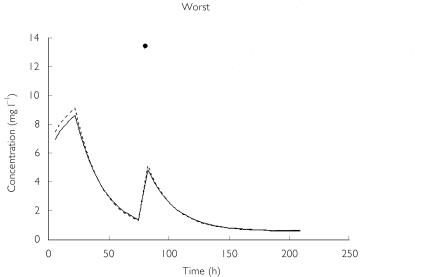
The predictive performance of the base model (clearance and volume modelled with weight only) when compared with the final model (the addition of age to clearance) further confirmed the superiority of the latter (Table 5). The bias of the model declined from 6.4% to 4.3%, whereas the precision improved from 30.6% to 27.6%. The worst and best predictive profiles from the final model are shown in Figure 4. The cross-validation analysis of the final model revealed estimates of structural parameters (and their CVs) comparable to those of the final model, suggesting that the model is likely to perform well in a truly prospective trial under similar conditions. The validation exercise revealed an increased bias of 9.2%, indicating that the model had a small tendency to over-predict. The predictive precision from the cross-validation was determined to be very similar at 29.5%.
Table 5.
Predictive performance and cross-validation
| Base model | Full model | Cross-validation | |
|---|---|---|---|
| Bias* (%) | 6.4 (−0.9, 16.3) | 4.3 (−6.6, 13.2) | 9.2 (2.9, 16.5) |
| Precision† (%) | 30.6 (26.6, 34.8) | 27.6 (22.2, 33.6) | 29.5 (24.8, 36.0) |
Bias (%) = median ([observedij-predictedij/predictedij]×100), i (ith individual), j (jth concentration).
Precision (%) = absolute median ([observedij-predictedij/predictedij]× 100), i (ith individual), j (jth concentration). Figures in parentheses are 95% confidence intervals for the median.
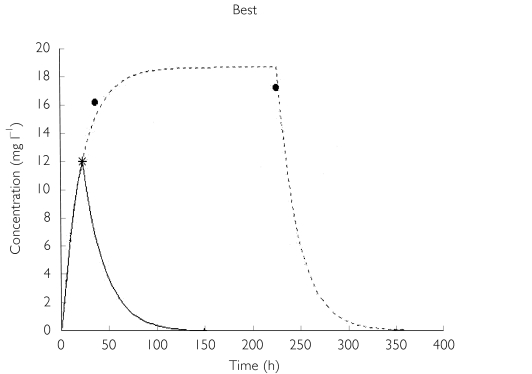
Figure 4.
The worst and best time-concentration profiles predicted by the final model. Worst: •, observed; —, predicted (P); —, predicted (I). Best (two individuals): --, predicted (1); *, observed (1); --, predicted (2); •, observed (2).
In the study by Pretzlaff et al., increased diuresis was observed at theophylline concentrations between 6 and 12 mg l−1, lower than that required in asthma therapy [4]. Thus, a proposed new dosing regimen to achieve average steady-state concentrations of 10 mg l−1 following a loading dose, was developed, based on calculations from the clearance and volume of distribution parameters determined in the final model (Table 6).
Table 6.
Maintenance doses of aminophylline (µg kg−1 min−1) for continuous infusion to achieve average steady-state concentration of 10 mg l−1
| Age (days) | 10th, 50th and 90thpercentile weight (kg) | Maintenance infusion rate (µg kg−1min−1) |
|---|---|---|
| 1 | 3 | 4.8 |
| 3.5 | 4.8 | |
| 4 | 4.8 | |
| 30 | 3.5 | 4.9 |
| 4.3 | 4.9 | |
| 5 | 4.9 | |
| 365 | 8.8 | 5.3 |
| 10 | 5.2 | |
| 11.5 | 5.2 | |
| 1095 | 12.9 | 5.8 |
| 14.5 | 5.7 | |
| 17 | 5.6 | |
| 1825 | 15.9 | 6.2 |
| 18.5 | 6.0 | |
| 21.5 | 5.8 | |
| 3650 | 25 | 6.5 |
| 30 | 6.2 | |
| 37 | 6.0 | |
| 5475 | 47 | 6.2 |
| 56 | 6.0 | |
| 68 | 5.7 |
Recommended maintenance infusion rates following an initial loading dose (0.57 × weight (kg) × 10 mg l −1). Maintenance infusion rate calculated from: average steady-state concentration = rate of infusion/clearance (using clearance parameters determined in the final model).
Discussion
ECMO patients, who comprise a group from the extreme spectrum of critical illness, are supported with intensive medical interventions and a myriad of pharmacological agents. However, there is a dearth of reported studies on altered drug disposition during ECMO to aid healthcare professionals [18]. The use of bolus doses of theophylline as a diuretic in ECMO neonates has previously been reported and results in an increased urine flow rate when compared with frusemide alone [3]. The present study provides the first data on the population pharmacokinetics of theophylline in paediatric patients receiving ECMO. In contrast to previous population studies, the pharmacokinetic model was developed through data obtained from continuous intravenous infusions of aminophylline.
As with previous studies, weight and age emerge as the most important factors affecting theophylline clearance [5–15]. Both these covariables are strong indicators of hepatic and renal maturation. However, in the study reported by Moore et al. clearance was modelled as a nonlinear exponential function of weight showing a disproportionate increase in the former with the latter [6]. Even though the present investigation included a wide weight and age range, the regression analysis suggested a linear relationship with clearance. Moreover, exponential functions of weight did not improve the model. The inclusion of age in the clearance model further improved the parameter estimate and reduced interpatient variability.
The estimated clearance of theophylline from this study appears to be significantly lower than in previous reports of similar age groups. Whereas previous neonatal studies involved premature infants of lower birth weights, neonates in this investigation were full term, of greater birth weights and would be expected to demonstrate greater clearance. However, values for the latter were similar to those found from traditional pharmacokinetic studies in premature neonates [9, 30–32]. Although previous population studies in premature infants by Lee et al. (mean weight 1.15 kg, mean gestational age 28.5 weeks) and Moore et al. (mean weight 1.5 kg, mean gestational age 31 weeks) have reported similar models, the differing neonatal populations make comparisons difficult [6, 8].
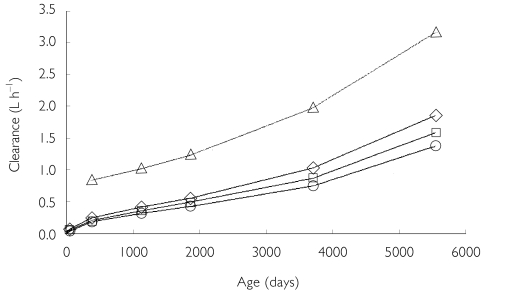
Our estimates of clearance in older infants and children are substantially lower than previous values (Figure 5) [7, 10, 12, 15]. There may be a number of reasons for this.
Figure 5.
A comparison of the estimated theophylline clearances based on age determined by the present study (at 10th (○), 50th (□) and 90th (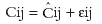 ) percentile weights) and a paediatric population study by Driscoll et al.[12] (▵)
) percentile weights) and a paediatric population study by Driscoll et al.[12] (▵)
Firstly, the volume in the ECMO circuit significantly expands the total circulating blood volume in the patient and therefore would be expected to increase the volume of distribution of theophylline (Table 1). In the present study, volume was modelled as a simple function of weight and the final estimate of 0.57 l kg−1 may be indicative of an enlarged volume of distribution in comparison with previous reports in similar age groups [7, 10, 15]. Secondly, the majority of our patients have significant and prolonged periods of hypoxia prior to cannulation for ECMO, often resulting in ischaemic insult to the kidneys and liver. This would potentially affect the excretion of unchanged drug to the urine (the main elimination route in neonates) and hepatic metabolism. Previous workers have reported significantly reduced theophylline clearance in neonates with hypoxia and birth asphyxia [9, 33, 34]. Unfortunately, it was not possible to test the influence of these covariables, as an index of hypoxia at or before cannulation was not available in many patients, nor was it possible to ascertain birth asphyxia in many of the neonates. There was also no discernible relationship between serum creatinine data and clearance. Ten patients (13.3%) in the study population required renal support in the form of CVVH, but no significant influence on the model was demonstrable. Third, although no overt manifestations of hepatic failure were seen, nine patients (12%) in the group were referred with congenital diaphragmatic hernia (CDH), a surgical condition known to alter hepatic blood flow [35]. Although this is most likely to affect those drugs with a high hepatic extraction ratio, significant changes in blood flow may also influence drugs of low to intermediate extraction such as theophylline [36]. No significant influence of patients with CDH was demonstrable. It is known that cardiac failure can significantly decrease theophylline clearance [37,38]. Twelve (16%) ECMO patients in this study were postoperative cardiac surgical patients, often requiring multiple pharmacological agents for cardiovascular support. The pharmacokinetics of theophylline in these patients did not differ from the rest of the population.
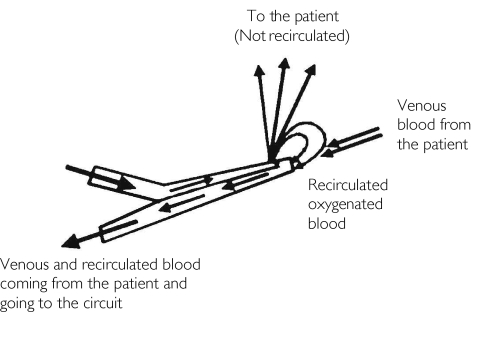
Finally, decreased clearance (and increased volume of distribution) may also be a consequence of the blood recirculation phenomena during VV ECMO (Figure 6). It is inevitable that a fraction of blood that has been oxygenated in the circuit flows directly from the reinfusion site to the drainage catheter and back into the circuit instead of the patient's circulation. The proportion of blood that recirculates is thought to depend on circuit pump flow, catheter position, cardiac output and right atrial size [39]. As drugs are administered into the circuit during ECMO, recirculation could significantly affect the removal of drugs. In addition, high flow rates during VA ECMO produce apulsatile blood flow that can also alter perfusion of tissues, reducing capillary circulation and aerobic metabolism [40]. Although it was not possible to show a significant influence of cannulation method on the model, there was a tendency towards an increased volume of distribution in the VV group.
Figure 6.
Recirculation in a double lumen veno-venous catheter
The ECMO population consists of patients with extremely severe pulmonary or cardiopulmonary disease as a result of wide-ranging primary diagnoses. The heterogeneous characteristics of our study population may help explain the wide interpatient variability estimated for clearance (39%) and volume (42%). The study population included patients with congenital and acquired respiratory failure (of an infective and noninfective aetiology), congenital cardiac defects, septicaemia, CDH, postoperative cardiac and thoracic cases. As described above, the influences of these subgroups in the model were not detected, probably as a result of limited numbers. Furthermore, as the ratio of extracorporeal : patient volume decreases in older children (see Table 1), this may have contributed to the increased variability in volume of distribution. Age did not significantly affect volume of distribution. However, there was a tendency towards a negative relationship with age. The precision with which the relationship was estimated was higher for clearance (25%) than volume (81%), as a large majority of the serum theophylline concentrations was sampled at steady state, of which the major influential pharmacokinetic parameter is clearance [41]. The estimate of residual error (3.6 mg l−1) is higher than previously reported and reflects model misspecification, sampling and dosing errors, as well as changing parameters during the period of analysis. The latter point may be particularly relevant during ECMO, as the recirculation phenomenon (affecting clearance and volume of distribution) would be expected to be less prevalent towards the end of ECMO treatment when the pump flow rates are significantly lower.
We evaluated the predictive accuracy and validity of our proposed final model using the cross-validation method. It is evident from the methodology described that cross-validation is a conservative measure of the expected performance, as each submodel is based on a reduced data set. The analysis revealed a greater bias than with the complete data set model, suggesting that the measured values tended to be on average slightly higher than predicted. There was no material change in the magnitude of the variability in the prediction error (27.6% vs. 29.5%), confirming that our final model shows reasonable precision.
It was not an aim of this investigation to define an aminophylline dose regimen for diuresis in paediatric ECMO patients. Ideally, a well-designed prospective pharmacokinetic-pharmacodynamic investigation can do this reliably. However, the results of the present investigation provide a valuable dosing guide. A large proportion (20%) of the measured plasma concentrations in this investigation were potentially toxic (>20 mg l−1), suggesting that the maintenance infusion dose rate should be decreased in these patients (Table 6). The long half-life in this study group (mean 16.6 h) suggests that an initial loading dose is necessary to achieve rapid and effective plasma concentrations.
In conclusion, this is the first report of population pharmacokinetics of theophylline in paediatric ECMO patients. The estimated clearance is significantly lower and the volume of distribution higher than previously reported in this age group. These differences are probably a result of the expanded circulating volume during ECMO, but also the extreme nature of critical illness in this group as well as altered renal and hepatic physiology. The large interindividual variability observed in theophylline pharmacokinetics reflects the heterogeneous nature of patients treated on ECMO. The validity of the derived pharmacokinetic model was substantiated using the cross-validation method.
References
- 1.Davis J, Shock N. The effect of theophylline ethylene diamine on renal function in control subjects and in patients with congestive heart failure. J Clin Invest. 1949;28:1459–1468. doi: 10.1172/JCI102211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sigurd B, Olesen K. The supra-additive natriuretic effect addition of theophylline ethylenediamine and bumetanide in congestive heart failure. Am Heart J. 1977;94:168–174. doi: 10.1016/s0002-8703(77)80276-7. [DOI] [PubMed] [Google Scholar]
- 3.Lochan S, Adeniyi-Jones S, Assadi F, Frey B, Marcus S, Baumgart S. Coadministration of theophylline enhances diuretic response to furosemide in infants during extracorporeal membrane oxygenation. A randomized controlled pilot study. J Pediatr. 1998;133:86–89. doi: 10.1016/s0022-3476(98)70183-0. [DOI] [PubMed] [Google Scholar]
- 4.Pretzlaff R, Vardis R, Pollack M. Aminophylline in the treatment of fluid overload. Crit Care Med. 1999;27:2782–2785. doi: 10.1097/00003246-199912000-00029. [DOI] [PubMed] [Google Scholar]
- 5.Nassif E, Weinberger M, Shannon D, Guiang S, Hendeles L, Jimenez D, et al. Theophylline disposition in infancy. J Pediatrics. 1981;98:158–161. [PubMed] [Google Scholar]
- 6.Moore E, Faix R, Banagale R, Grasela T. The population pharmacokinetics of theophylline in neonates and young infants. J Pharmacokinetics Biopharmaceutics. 1989;17:47–66. doi: 10.1007/BF01059087. [DOI] [PubMed] [Google Scholar]
- 7.Loughnan P, Sitar D, Ogilvie R, Eisen A, Fox Z, Neims A. Pharmacokinetic analysis of the disposition of intravenous theophylline in young children. J Pediatr. 1976;88:874–879. doi: 10.1016/s0022-3476(76)81136-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee T, Charles B, Steer P, Flenady V, Grant T. Theophylline population pharmacokinetics from routine monitoring data in very premature infants with apnoea. Br J Clin Pharmacol. 1996;41:191–200. doi: 10.1111/j.1365-2125.1996.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 9.Gilman J, Gal P, Levine R, Hersh C, Vildan Erkan N. Factors influencing theophylline disposition in 179 newborns. Therapeutic Drug Monitoring. 1986;8:4–10. doi: 10.1097/00007691-198603000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Elliott E, Koysooko R, Levy G. Pharmacokinetics of theophylline in children with asthma. Pediatrics. 1976;58:542–547. [PubMed] [Google Scholar]
- 11.du Preez M, Botha J, McFayden M, Holford N. The pharmacokinetics of theophylline in premature neonates during the first few days after birth. Therapeutic Drug Monitoring. 1999;21:598–603. doi: 10.1097/00007691-199912000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Driscoll M, Ludden T, Casto D, Littlefield L. Evaluation of theophylline pharmacokinetics in a pediatric population using mixed effects models. J Pharmacokinetics Biopharmaceutics. 1989;17:141–168. doi: 10.1007/BF01059025. [DOI] [PubMed] [Google Scholar]
- 13.Aranda J, Sitar D, Parsons W, Loughnan P, Neims A. Pharmacokinetic aspects of theophylline in premature newborns. N Engl J Med. 1976;295:413–416. doi: 10.1056/NEJM197608192950803. [DOI] [PubMed] [Google Scholar]
- 14.Karlsson M, Thomson A, McGovern E, Chow P, Evans T, Kelman A. Population pharmacokinetics of rectal theophylline in neonates. Therapeutic Drug Monitoring. 1991;13:195–200. doi: 10.1097/00007691-199105000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Rosen J, Danish M, Ragni M, Lopez Saccar C, Yaffe S, Lecks H. Theophylline pharmacokinetics in the young infant. Pediatrics. 1979;64(2):248–251. [PubMed] [Google Scholar]
- 16.Peek G, Killer H, Sosnowski A, Firmin R. Extracorporeal membrane oxygenation: potential for adults and children? Hospital Med (London) 1998;59:304–308. [PubMed] [Google Scholar]
- 17.Bartlett R, Roloff D, Custer J, Younger J, Hirschl R. Extracorporeal life support. The University of Michigan Experience. J Am Med Assoc. 2000;283:904–908. [PubMed] [Google Scholar]
- 18.Mulla H, Lawson G, Firmin R, Upton D. Drug disposition during extracorporeal membrane oxygenation (ECMO) Paediatric Perinatal Drug Ther. 2001;4:109–120. [Google Scholar]
- 19.Mulla H, Lawson G, von Anrep C, Burke M, Upton D, Firmin R, et al. In vitro evaluation of sedative drug losses during extracorporeal membrane oxygenation. Perfusion. 2000;15:21–26. doi: 10.1177/026765910001500104. [DOI] [PubMed] [Google Scholar]
- 20.Cohen P, Collart L, Prober C, Fischer A, Blaschke T. Gentamicin pharmacokinetics in neonates undergoing extracorporeal membrane oxygenation. Paediatric Infect Dis. 1990;9:562–566. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Sheiner L, Rosenberg B, Arathe V. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinetics Biopharmaceutics. 1977;5:445–479. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- 22.Sheiner L, Grasela T. An introduction to mixed effect modelling. concepts, definitions, and justification. J Pharmacokinetics Biopharmaceutics. 1991;19(Suppl):11S–24S. June. [Google Scholar]
- 23.Pharsight. Winnonmix Professional. 2.0.1. Mountain View, CA: Pharsight Corp; 1993–2000. [Google Scholar]
- 24.Compaq. Compaq Visual Fortran Professional Edition. 6.5. Houston: Compaq Computer Corp; 2000. [Google Scholar]
- 25.Sun H, Fadiran E, Jones C, Lesko L, Huang S-M, Higgins K, et al. Population pharmacokinetics: a regulatory perspective. Clin Pharmacokinetics. 1999;37:41–58. doi: 10.2165/00003088-199937010-00003. [DOI] [PubMed] [Google Scholar]
- 26.Fiset P, Mathers L, Engstrom R, Fitzgerald D, Brand S, Hsu F, et al. Pharmacokinetics of computer-controlled alfentanil administration in children undergoing cardiac surgery. Anesthesiology. 1995;83:944–955. doi: 10.1097/00000542-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Zomorodi K, Donner A, Somma J, Barr J, Sladen R, Ramsay J, et al. Population pharmacokinetics of midazolam administered by target controlled infusion for sedation following coronary artery bypass grafting. Anesthesiology. 1998;89:1418–1429. doi: 10.1097/00000542-199812000-00020. [DOI] [PubMed] [Google Scholar]
- 28.Kerbusch T, Kraker JD, Mathot R, Beijnen J. Population pharmacokinetics of ifosfamide and its dechloroethylated and hydroxylated metabolites in children with malignant disease: a sparse sampling approach. Clin Pharmacokinetics. 2001;40:615–625. doi: 10.2165/00003088-200140080-00005. [DOI] [PubMed] [Google Scholar]
- 29.Sheiner L, Beal S. Some suggestions for measuring predictive performance. J Pharmacokinetics Biopharmaceutics. 1981;9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 30.Brazier J, Renaud H, Ribon B, Salle B. Serum xanthine levels in low birthweight infants treated or not treated with theophylline. Arch Dis Childhood. 1979;54:194–199. doi: 10.1136/adc.54.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones R, Baillie E. Dosage schedule for intravenous aminophylline in apnoea of prematurity, based on pharmacokinetic studies. Arch Dis Childhood. 1979;54:190–193. doi: 10.1136/adc.54.3.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilligross D, Jusko W, Koup J, Giacoia G. Factors affecting theophylline pharmacokinetics in premature infants with apnoea. Dev Pharmacol Therapeutics. 1980;1:6–15. [PubMed] [Google Scholar]
- 33.Estelle F, Simons R, Rigatto H, Simons K. Pharmacokinetics of theophylline in neonates. Semin Perinatol. 1981;5:337–345. [PubMed] [Google Scholar]
- 34.Gal P, Boer H, Toback J, Wells T, Erkan N. Effect of asphyxia on theophylline clearance in newborns. Southern Med J. 1982;75:836–838. doi: 10.1097/00007611-198207000-00017. [DOI] [PubMed] [Google Scholar]
- 35.MacGillivray T, Jennings R, Rudolph A, Ring E, Adzick N, Harrison M. Vascular changes with in utero correction of diaphragmatic hernia. J Pedia Surg. 1994;29:992–996. doi: 10.1016/0022-3468(94)90265-8. [DOI] [PubMed] [Google Scholar]
- 36.Mckindley D, Hanes S, Boucher B. Hepatic drug metabolism in critical illness. Pharmacotherapy. 1998;18:759–778. [PubMed] [Google Scholar]
- 37.Jusko W, Gardner M, Mangione A, Schentag JJ, Koup JR, Vance JW. Factors affecting theophylline clearance: age, tobacco, marijuana, cirrhosis, congestive heart failure, obesity, oral contraceptives, benzodiazepines, barbiturates, and ethanol. J Pharmaceut Sci. 1979;68:1358–1366. doi: 10.1002/jps.2600681106. [DOI] [PubMed] [Google Scholar]
- 38.Self T, Chafin C, Soberman J. Effect of disease states on theophylline serum concentrations: are we still vigilant? Am J Med Sci. 2000;319:177–182. doi: 10.1097/00000441-200003000-00007. [DOI] [PubMed] [Google Scholar]
- 39.Zwischenberger JB, Steinhorn RH, Bartlett RH, editors. ECMO: Extracorporeal Cardiopulmonary Support in Critical Care. 2. Ann Arbor, MI: Extracorporeal Life Support Organization; 2000. [Google Scholar]
- 40.Shevde K, Dubois W. Pro: pulsatile flow is preferable to nonpulsatile flow during cardiopulmonary bypass. J Cardiothoracic Anaesth. 1987;1:165–168. doi: 10.1016/0888-6296(87)90012-3. [DOI] [PubMed] [Google Scholar]
- 41.Rowland M, Tozer T. Clinical Pharmacokinetics: Concepts and Applications. 3. Philadelphia: Lippincott Williams & Wilkins; 1995. [Google Scholar]
- 42.Textbook of Paediatrics. 3. Edinburgh: Churchill Livingstone; 1984. [Google Scholar]