HIV therapy
In the late 1980s Alasdair Breckenridge had the foresight to see that the fast-tracking of drugs for the treatment of human immunodeficiency virus (HIV) infection left many issues relating to both efficacy and toxicity of these drugs unanswered. The Department of Pharmacology and Therapeutics at Liverpool had considerable expertise in drug metabolism and pharmacokinetics, and there was the potential to make a real impact in this area. The Liverpool HIV Pharmacology Group was soon established and has been recognized as one of the leading centres in the world for this area of research.
HIV infection has already claimed the lives of more than 20 million individuals, mostly within sub-Saharan Africa. As of mid-2002, more than 40 million are currently infected with the virus. During the last 20 years there has been remarkable progress in the therapy of HIV disease. At the beginning of the pandemic all that was offered was prophylaxis and treatment of opportunistic infections. The first drug to be licensed for activity against HIV was the nucleoside reverse transcriptase inhibitor (NRTI), zidovudine. From those early days of using single drugs (monotherapy), which gave only a transient change in viral load, we moved to dual therapy (two NRTIs) and then in the mid-1990s to triple or quadruple therapy (highly active antiretroviral therapy; HAART).
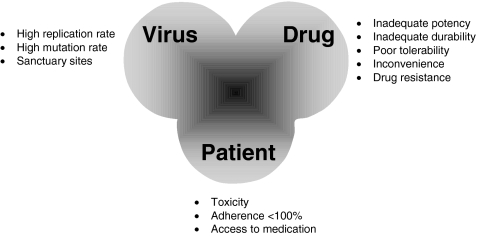
HAART can suppress viral replication and substantially prolong patient life [1]. It can also fail for a number of reasons, including poor adherence, insufficient drug potency, emergence of resistance, cellular factors, and pharmacokinetic factors (Figure 1). Although many antiretroviral drugs are now available, a limited number of combinations have been proven effective for individual patients. With sequential treatment failures, the durability of virological response tends to decrease with subsequent treatment regimens until the patient is left with few or no therapeutic options [2]. There is evidence that many treatment-naïve patients will switch from their initial regimen within 1 year. It is imperative that we adopt strategies that will optimize the use of available therapies, so as to achieve long-term viral suppression.
Figure 1.
Reasons for failure of antiretroviral therapy
HAART comprises ≥ three drugs in combination from the following classes: NRTIs, non-nucleoside reverse transcriptase inhibitors (NNRTI) and protease inhibitors (PI) (Table 1. NRTIs are pro-drugs, requiring intracellular conversion to their active drug-triphosphate metabolites (which compete with endogenous nucleotides) in order to inhibit HIV replication. In contrast, PIs act directly to inhibit HIV protease, and are extensively metabolized by the cytochrome P450 isoform CYP3A4. The likelihood of treatment success in patients on HAART is directly related to exposure of the virus to active drug. Plasma PI (and probably NNRTI) concentrations are predictive of treatment failure [3] and may also predict toxicity (e.g. with ritonavir, indinavir, nevirapine and efavirenz [3, 4]). This brief review focuses on two current strategies where the aim is the optimization of antiretroviral therapy in infected patients. The first strategy is pharmacokinetic and involves drug monitoring, and as indicated below this is being introduced into clinical practice. The second strategy is to seek to understand the role of pharmacogenomics in the variability in plasma drug concentrations and therefore in drug response.
Table 1.
Current anti-HIV therapy.
| Nucleoside analogues | Protease inhibitors |
| Zidovudine (ZDV) | Saquinavir (SQV) |
| Zalcitabine (ddC) | Ritonavir (RTV) |
| Lamivudine (3TC) | Indinavir (IDV) |
| Didanosine (ddI) | Nelfinavir (NFV) |
| Stavudine (d4T) | Amprenavir (APV) |
| Abacavir (ABC) | Lopinavir (LPV) |
| Non-nucleoside RT inhibitors | Nucleoside RT inhibitor |
| Nevirapine | Tenofovir |
| Delavirdine | |
| Efavirenz |
Therapeutic drug monitoring
Monitoring the course of HIV infection has been an essential component of patient management. CD4 counts help track the immunological status of a patient, and viral-load assays are used to monitor the antiviral activity and durability of a regimen, and to guide treatment changes. Resistance assays are becoming a standard element of care despite issues regarding interpretation of results. With emerging evidence linking drug exposure to both antiviral efficacy and toxicity, attention is now being focused on the role of monitoring plasma drug concentrations in patients receiving HAART. If inadequate drug concentrations, arising from poor adherence, inherent pharmacokinetic factors or drug interactions, are a major cause of treatment failure, then monitoring these concentrations and having an intervention strategy seems a logical approach to improve the success rate of therapy. Likewise, high drug concentrations may relate to toxicity, either in the patient at that time or somewhere down the line.
Managing the therapeutic regimen of a patient on the basis of measured drug concentrations is referred to as Therapeutic Drug Monitoring (TDM). In virtually all cases, TDM is applied to drugs exhibiting a narrow therapeutic window, in order to optimize efficacy and/or avoid toxicity. This diagnostic approach has been used for many years with anticonvulsants, immunosuppressants, aminoglycoside antibiotics, and some cytotoxics. In the setting of HIV management the potential role of TDM is receiving increasing attention, and there are clearly several features of current antiretroviral therapy that suggest that TDM may have benefit. As pointed out by Flexner & Piscitelli [5], the single most important aspect of HIV therapy that draws us to consider the role of TDM is the fact that treatment is lifelong in most cases. We still have relatively few options despite the 16 licensed antiretrovirals, so getting it right (ensuring there is enough drug for efficacy but not too much for toxicity) is surely a major goal. Care providers need to know that drug concentrations achieved in a patient have a high probability of chronically suppressing HIV replication without generating drug resistance or problematic toxicities. They also need to remember that drug concentrations may more accurately correlate acute, rather than chronic or cumulative toxicities.
A major confounder when considering TDM in a clinical setting is adherence. Patients who do not take their antiretroviral therapy on schedule or do not comply with food requirements would be expected to have low plasma drug concentrations and consequently, a poor outcome. However, those patients may have apparently ‘normal’ plasma concentrations if they take the dose just before a hospital visit. A patient failing therapy for poor adherence would appear to have a normal drug concentration in any outcome analysis. Hence a thorough assessment of adherence is essential within any TDM programme to facilitate interpretation of concentrations and to ensure good outcome. It is also critical to know the accurate timing of a blood sample in relation to when the dose was taken.
Another important consideration in TDM is setting the target drug concentrations. There is some degree of confidence that we know minimum effective concentrations (MECs), values that are generated and extrapolated from patient studies or in vitro studies, at least in treatment-naïve patients. There is much less confidence surrounding maximum concentrations, with the exception of indinavir and possibly efavirenz. The presence of drug-resistant virus for an antiretroviral drug means that it is not rational to use the same set of target concentrations for patients harbouring drug-sensitive and drug-resistant virus. This is where relating drug concentrations to the patient viral isolate becomes a much more attractive option than TDM on its own. The use of inhibitory quotients (IQ), virtual IQ (vIQ) or normalized IQ (nIQ) (Figure 2) is currently being clinically evaluated for a number of different drugs. Data presented during 2002 [6, 7] gives grounds for optimism that this approach may prove of considerable benefit, and probably better than TDM on its own. There is a price to pay (literally) for the advance in technology, and the issue of reimbursement is likely to prove a considerable stumbling block to the widespread access to the test. Clearly what we need is good economic evaluation of the cost-benefit factors surrounding the use of TDM with or without resistance testing. In absolute costs TDM is ‘cheap’ (£40 per sample), compared with resistance testing (genotype £200, virtual phenotype £280).
Figure 2.
Definitions of inhibitory quotient (IQ) and virtual inhibitory quotient (vIQ) bringing together the drug concentration and a pharmacodynamic measure in a simple equation IQ = Ctrough/IC50, where Ctrough is the drug concentration at the end of the dosing interval and IC50 is the concentration of drug producing 50% inhbition of virus in vitro. vIQ = Ctrough/IC50 of WT virus x Virtual Phenotype (VP), where the VP is determined from the genotype and matched phenotype in a data base.
Defining the clinical situations in which TDM will have the greatest use is a priority. The recent interim analysis of the ATHENA trial [8] has suggested that dose adjustment of indinavir reduced toxicity and maintained patients on the indinavir-containing regimen. On the other hand, TDM of nelfinavir identified patients with low plasma concentrations. By either ensuring the drug was taken with food or dose modification, the TDM arm showed better virological outcome at 12 months.
Despite the lack of randomized controlled trial data, there are probably a number of other specific situations in addition to indinavir and nelfinavir that warrant TDM. The use of lopinavir plus ritonavir (Kaletra) with am-prenavir gives rise to a complex interaction of three drugs, and monitoring the concentrations of both lopinavir and amprenavir may be important. The use of once-daily regimens can also lead to trough plasma concentrations that are close to the MEC, which is true of saquinavir plus ritonavir. Thus TDM will identify any individuals with low and potentially problematic concentrations.
Certain patient groups are clearly at increased risk for unpredictable and potentially damaging pharmacokinetic profiles, and could benefit from TDM. This includes patients with liver or renal damage, paediatric and pregnant patients, and patients with complex drug interactions. When putting a TDM scheme into practice, it is important to have a clearly defined procedure for modifying the dose, especially as it may take some time for plasma concentrations to reach steady state following any dose modification. A practical approach for this is to derive a treatment algorithm that lists the reasons why a patient may enter a TDM programme and the potential results, and provides guidance on how therapy should be modified. If TDM is to have a greater role in antiretroviral therapy, more research, including large randomized trials to assess its clinical utility and routine applicability, is needed. An international consensus must be reached on target concentrations and the tests used. In addition, standardization between centres is needed on units of measurement, values for virtual phenotypes, and the way results are presented. Much remains to be learned, and there are a number of challenges facing the introduction of TDM into clinical practice. If we can benefit some patients by ensuring efficacy or limiting toxicity simply by taking a couple of extra blood samples, we should vigorously standardize and validate this approach.
Pharmacogenomics
Pharmacogenomics has been widely hailed as a means to improve prescribing for all drugs. However, in particular, pharmacogenomics is likely to be useful for drugs with variable pharmacokinetics and a relatively narrow therapeutic index. As described above, certain anti-HIV drugs fit this category and consequently there is a growing interest in the possibility of individualizing antiretroviral therapy through the use of pharmacogenomics.
Following the administration of standard doses of an antiretroviral drug, large (approximately 100-fold) interindividual variability is consistently observed in plasma concentrations of protease inhibitors and non-nucleoside reverse transcriptase inhibitors [1] as well as intracellular NA-triphosphates [9]. Much interest has focused around the influence of host genetic polymorphism upon interindividual variability in drug kinetics and treatment response. Candidates include drug-metabolizing enzymes such as cytochrome P450 CYP 3A5, 2D6, 2C9 and 2C19 [10]. Although polymorphisms in the P450 genes have attracted most attention, it is important to note that phase II enzymes are also involved in the metabolism of antiretrovirals. Thus, polymorphisms in genes coding for phase II enzymes such as glucuronyl transferase may also be important.
The binding of basic drugs to α1-acid glycoprotein (AGP) has previously been recognized to differ between individuals according to protein isoform as distinguished by isoelectric focusing. The genetic basis of the prevailing three common alleles has been mapped to single nucleotide polymorphisms (SNPs) within exons 1 and 5 in the ORM1 gene locus [11]. Genetic polymorphisms have also been described for the drug efflux transporter p-glycoprotein (P-gp; MDR1 gene; ABCB1 gene). Although many SNPs have been described within MDR1, a C→T transition at position 3435 (exon 26) was significantly associated with plasma digoxin concentrations [12]. Data from the Swiss Cohort Study in collaboration with the Liverpool Group [13] have also demonstrated a significant association between certain MDR-1 genotypes and plasma concentrations of nelfinavir and efavirenz, as well as subsequent CD4 count rise upon initiation of antiretroviral therapy. The study further revealed that the effect of genetic variability upon drug disposition and metabolism is complex, and may relate to expression of P-gp at different sites as well as secondary effects of other metabolic factors such as cytochrome P450.
Another important cellular protein is PXR (pregnane X receptor), a xenobiotic-regulated transcription factor that co-ordinately regulates transcription of CYP3A4 and MDR1. Like its target genes, PXR displays a broad specificity for a variety of drugs including protease inhibitors. PXR appears to be a master regulator of drug clearance, and functional variants are likely to have significant effects on drug concentrations. Recent data [14] have shown a number of SNPs in the coding region, of which three are nonsynonymous, thereby creating new PXR alleles. Of some importance is the finding that the frequency of PXR*2 was 0.20 in African Americans and was not found in Caucasians. As ligand activation of PXR and upregulation of other genes are major determinants of drug disposition, it is important to relate PXR genotype to plasma concentrations of antiretrovirals and efficacy of antiretroviral therapy.
Apart from P-gp, genetic polymorphisms have not been well characterized at other transporter loci. However, SNPs have been reported in pooled scientific databases (e.g. The SNP Consortium) for the multidrug-resistance proteins (MRP-1, MRP-2, MRP-5), organic anion transporter protein (OATP), and the human equilibrative nucleoside transporter (hENT2) (see http://www.snp.cshl.org). No allele frequencies are available. The Liverpool HIV Pharmacology Group, in collaboration with the Centre for Integrated Genomic Medical Research at Manchester University, is currently undertaking work to validate these SNPs (focusing particularly on those located within coding regions of the gene), and to define common haplotypes for each drug transporter.
Improvement in HAART through the application of pharmacogenomics is going to be a complex process. The genomics of both the virus and the host need to be considered to ensure the meaningful application of genotypic guided therapy. It is also important to note that when a genetic determinant of efficacy is identified in one ethnic population, it may not necessarily be relevant in another ethnic population because of (sometimes marked) ethnic variations in the frequency of polymorphisms. In order to unravel the complexity of HIV pharmacogenomics, a multifaceted approach will be essential.
It is hoped that a better understanding of pharmacogenetics in relation to drug disposition will have direct relevance to the treatment of HIV-infected patients and ultimately help in tailoring antiretroviral therapy to an individual patient for optimal efficacy and safety.
References
- 1.Palella F, Chmiel J, Moorman AC, et al. Durability and predictors of success of highly active antiretroviral therapy for ambulatory HIV-infected patients. AIDS. 2002;16:1617–1626. doi: 10.1097/00002030-200208160-00007. [DOI] [PubMed] [Google Scholar]
- 2.Ledergerber B, Egger M, Opravil M, et al. Clinical progression and virological failure on highly active anti-retroviral therapy in HIV-1 patients: a prospective cohort study. Lancet. 1999;353:863–868. doi: 10.1016/s0140-6736(99)01122-8. [DOI] [PubMed] [Google Scholar]
- 3.Back DJ, Gatti G, Fletcher C, et al. Therapeutic drug monitoring in HIV infection: current status and future directions. AIDS. 2002;16(Suppl 1):S5–S37. doi: 10.1097/00002030-200203001-00002. [DOI] [PubMed] [Google Scholar]
- 4.van Heeswijk RPG. Critical issues in therapeutic drug monitoring of antiretroviral drugs. Ther Drug Monit. 2002;24:323–331. doi: 10.1097/00007691-200206000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Flexner CW, Piscitelli SC. Concentration-targeted therapy and the future of HIV management. AIDS. 2002;16(Suppl 1):S1–S3. doi: 10.1097/00002030-200203001-00001. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher CV, Cheng H, Fiscus S, et al. Ninth Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2002. The inhibitory quotient (IQ) for saquinavir (SQV) predicts virologic response to salvage therapy; pp. 24–28. February, [Abst.129] [Google Scholar]
- 7.Phillips E, Tseng A, Walker S, et al. Ninth Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2002. The use of virtual inhibitory quotient (vIQ) in antiretroviral (ART) -experienced patients taking amprenavir/lopinavir combinations; pp. 24–28. February, [Abstr.130] [Google Scholar]
- 8.Burger D. First IAS Conference on HIV Pathogenesis and Treatment. Buenos Aries, Argentina: 2001. Therapeutic drug monitoring: A must for clinical practice? pp. 8–11. July, [Abstr. 54] [Google Scholar]
- 9.Hoggard PG, Kewn S, Lloyd J, et al. Ninth Conference on Retroviruses and Opportunistic Infections. Seattle, WA: 2002. Virological failure is associated with decreased 3TC triphosphate (3TCTP) and ratio of 3TCTP/endogenous deoxycytidine triphosphate in HIV-infected individuals; pp. 24–28. February, [Abstr.455] [Google Scholar]
- 10.Pirmohamed M, Back DJ. The pharmacogenomics of HIV therapy. The Pharmacogenomics J. 2001;1:243–253. doi: 10.1038/sj.tpj.6500069. [DOI] [PubMed] [Google Scholar]
- 11.Yuasa I, Umetsu K, Vogt U, et al. Human orosomucoid polymorphism: molecular basis of the three common ORM1 alleles, ORM1*F1, ORM1*F2, and ORM1*S. Hum Genet. 1997;99:393–398. doi: 10.1007/s004390050378. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmeyer S, Burk O, von Richter O, et al. Functional polymorphisms of the human multidrug resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci USA. 2000;97:3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellay J, Marzolini C, Meaden ER, et al. Response to antiretroviral treatment in HIV-1 infected individuals with allelic variants of the multidrug resistance transporter 1: a pharmacogenetics study. Lancet. 2002;359:30–36. doi: 10.1016/S0140-6736(02)07276-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhang J, Kuehl P, Green ED, et al. The human pregnane X receptor: genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics. 2001;11:551–552. doi: 10.1097/00008571-200110000-00003. [DOI] [PubMed] [Google Scholar]