Abstract
Aims
To evaluate the systemic bioactivity of triamcinolone acetonide (TA) 220 µg or mometasone furoate (MF) 200 µg over 3 weeks in perennial allergic rhinitis.
Methods
Twenty-seven patients received TA 220 µg or MF 200 µg once daily for 3 weeks with a 2 week placebo washout period prior to each randomized treatment. Measurements were made at baseline after each washout and after each randomized treatment, comprising overnight 10-h urinary cortisol corrected for creatinine (OUCC), 08.00 h plasma cortisol and 08.00 h serum osteocalcin.
Results
There were no significant differences between baseline values prior to TA or MF, and for any outcome measures comparing randomized treatments to respective baseline values or comparing TA with MF. For OUCC compared with baseline, the geometric mean fold suppression (95% CI) was 1.02 (0.78, 1.33) for TA (2% decrease), 1.07 (0.80, 1.42) for MF (7% decrease), and 1.05 (0.79, 1.39) for TA vs MF (5% decrease).
Conclusions
Standard doses of TA or MF over 3 weeks showed no differences in systemic bioactivity markers compared with respective baseline values after placebo washout, and there were no differences between TA vs MF.
Keywords: intranasal glucocorticoids, mometasone furoate, perennial allergic rhinitis, systemic bioactivity, triamcinolone acetonide
Introduction
Intranasal glucocorticosteroids (INS) are effective in treating allergic rhinitis and their systemic bioactivity can be assessed through suppression of the hypothalamic–pituitary–adrenal axis [1] by measuring early morning plasma cortisol and overnight 10-h urinary cortisol corrected for creatinine. We have previously shown that neither triamcinolone acetonide (TA) nor mometasone furoate (MF) produced significant systemic suppression of either adrenal or bone markers in patients with seasonal allergic rhinitis [2]. However in that study, INS were given for a 5-day period and it was not appreciated at the time that prolonged treatment for at least 2 weeks may be necessary in order to achieve true steady-state blood concentrations for MF [3]. It was therefore considered important to conduct a study with longer periods of INS treatment in order to achieve steady-state concentrations with MF and also to evaluate patients with perennial as opposed to seasonal allergic rhinitis.
Methods
Patients
Thirty patients with perennial allergic rhinitis were recruited. All patients had a positive skin prick test to house dust mite and one other common aero-allergen (grass pollen, cat, dog or mould). Significant nasal septal deviation of more than 50% and nasal polyposis were excluded by rhinoscopy. Patients who were on current therapy with INS (n = 13) had treatment discontinued during a 2-week washout period. In order to grade severity, patients completed a one-off total nasal symptom score sheet on study entry, based on scores of 1 (symptoms absent) to 5 (symptoms severe) for each of the five symptoms of runny nose, blocked nose, itchy nose, sneezing and general well-being over the previous week. Patients also recorded their peak nasal inspiratory flow using the In-Check® domiciliary nasal peak inspiratory flow meter (Clement Clarke International Ltd, Harlow, UK). However, clinical efficacy was not measured as an outcome in our study. All patients gave informed consent, and approval for the study was obtained from the Tayside Medical Ethics Committee.
Study design
An investigator-blind, randomized, crossover design was used. Patients were randomized to receive 3 weeks of either intranasal TA 220 µg (two sprays per nostril) once (08.00 h) daily (Nasacort® AQ, 55 µg per actuation; Aventis Pharma Ltd, West Malling, UK) or intranasal MF 200 µg (two sprays per nostril) once (08.00 h) daily (Nasonex®, 50 µg per actuation; Schering-Plough Ltd, Welwyn Garden City, UK). Patients had a 2 week washout period with intranasal placebo before each randomized treatment block. All patients were instructed on the correct technique of using the nasal sprays and were also given written instructions. Patients’ techniques were assessed and the correct method further re-emphasized at each study visit.
Measurements
Patients attended the laboratory at 07.30 h and rested in a supine position for 30 min. Following this, patients had blood samples taken for plasma cortisol along with serum osteocalcin at 08.00 h, and voided their bladder for the last time. The total collected urine thus represented an overnight 10 h excretion (22.00 h−08.00 h) with patients having last emptied their bladder at 22.00 h the night before. After recording of collected urinary volume, specimen aliquots were kept for cortisol and creatinine assay. Clinical efficacy was not measured as an outcome.
Assays
Assays were carried out by a technician in a blinded fashion. A commercial radioimmunoassay kit (DiaSorin Ltd, Workingham, UK) was used to measure plasma and urinary cortisol. There was no cross-reactivity with TA or MF. For plasma cortisol, the coefficient of variation for analytical imprecision was 4.8% and 5.6% for within assays and between assays, respectively. The coefficient of variation for urinary-free cortisol excretion was 7.7% within assays and 7.3% between assays. A Cobas-bio autoanalyser (Roche Products Ltd, Welwyn Garden City, UK) and kit (Sigma-Aldrich Co Ltd, Poole, UK) were used to measure urinary creatinine concentration with a coefficient of variation of 1.7% for within assays and 4.2% between assays. A radioimmunoassay kit (DiaSorin Ltd, Workingham, UK), which had a within-assay coefficient of variation of 7.8%, was used to measure serum osteocalcin.
Statistical analysis
The study was designed with a sample size of 20, allowing 80% power (α error = 0.2) to detect a 30% difference in OUCC (the primary end point) between each randomized treatment and its respective baseline, with α error set at 0.05 (two-tailed). To normalize distribution, all data including secondary end points (plasma cortisol and serum osteocalcin) were logarithmically transformed and analysed using a Statgraphics software package (STSC Software Group, Rockville, USA). Comparisons were made of the randomized treatments (TA and MF) and the respective baselines (prior to each treatment period) by an overall analysis of variance. To obviate multiple pair-wise comparisons, multiple-range testing with Bonferroni's correction was applied, set with 95% confidence intervals (P < 0.05, two-tailed).
Results
Twenty-seven patients (8F, 19M) of 37 ± 2 years (mean age ± SEM) with perennial allergic rhinitis completed the study. At initial screening, patients had a total nasal symptom score (out of 50) of 21 ± 2 and nasal peak inspiratory flow of 120 ± 11 l min−1. For all the end points, there were no significant differences between baseline values prior to randomized active treatments, no differences between randomized treatments vs their respective baselines, and no differences between randomized treatments as change from respective baselines (Table 1). For the primary outcome of OUCC compared with baseline, the geometric mean fold suppression (95% CI) was 1.02 (0.78, 1.33) for TA (2% decrease), 1.07 (0.80, 1.42) for MF (7% decrease), and TA vs MF 1.05 (0.79, 1.39) (Figure 1). For 08.00 h plasma cortisol vs baseline, the values were 0.99 (0.83, 1.19) for TA (1% increase), 1.04 (0.90, 1.20) for MF (4% decrease), and TA vs MF 1.05 (0.91, 1.21) and for 08.00 h serum osteocalcin vs baseline, 0.99 (0.67, 1.47) for TA (1% increase), 0.95 (0.67, 1.36) for MF (5% increase), and TA vs MF 0.96 (0.76, 1.21).
Table 1.
Geometric means (s.e. mean) for triamcinolone acetonide (TA) with respective baseline (TA–PL) and mometasone furoate (MF) with respective baseline (MF-PL).
| TA–PL | TA | MF–PL | MF | |
|---|---|---|---|---|
| Overnight urinary cortisol (nmol 10 h−1) | 35.0 (2.6) | 37.5 (3.7) | 51.2 (7.1) | 44.4 (4.4) |
| Overnight urinary cortisol : creatinine ratio (nmol mmol−1) | 7.8 (0.7) | 7.7 (0.7) | 9.6 (1.1) | 8.9 (0.8) |
| 08.00 h plasma corstisol (nmol l−1) | 450.8 (38.0) | 448.4 (28.0) | 446.9 (22.9) | 429.9 (24.5) |
| 08.00 h serum osteocalcin (nmol l−1) | 1.08 (0.15) | 1.08 (0.13) | 1.15 (0.14) | 1.19 (0.13) |
There was no significant difference between baselines prior to TA and MF, between each active treatment and respective baseline, and between active treatments as change from respective baselines.
Figure 1.
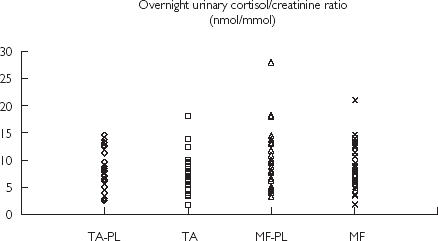
Individual values for triamcinolone acetonide (TA) with respective baseline (TA–PL) and and mometasone furoate (MF) with respective baseline (MF–PL) for overnight urinary cortisol : creatinine ratio (nmol mmol−1)
Discussion
From our results, there were no significant differences between each active treatment and their respective baselines after washout for all outcome measures. This was also the case for the comparison between TA and MF.
We incorporated a nonrandomized 2-week placebo washout period prior to each randomized treatment, which showed no differences for the primary outcome measure. The respective confidence intervals for within patient differences between randomized treatments and placebo baselines all included unity. We felt that a randomized placebo period was not essential in this study, as we did not measure efficacy, but only systemic bioactivity markers as such. Indeed, we had genuine ethical concerns regarding the use of a further 3-week randomized placebo plus a further 2-week washout in symptomatic patients with perennial allergic rhinitis, as this would have resulted in a high dropout rate, and would have dramatically reduced the number of potential volunteers.
MF is an intranasal corticosteroid that is highly lipophilic and exhibits a large volume of distribution with extensive extravascular tissue binding. It is therefore found in high concentrations in the fat soluble systemic tissue compartment with lower concentrations in the water soluble blood compartment [4]. Furthermore, it has a prolonged elimination due to equilibration between the tissue and blood compartments [5]. In contrast, less lipophilic corticosteroids such as TA exhibit preferential partitioning into the blood as compared with the systemic tissue compartment, resulting in a smaller volume of distribution and a shorter elimination half-life [6]. Our data appear to indicate that differences in bioavailability after a single dose [7] do not seem to predict systemic bioactivity after chronic dosing.
We chose fractionated OUCC as our primary outcome variable, as this is as sensitive as an integrated blood or urine 24-h cortisol profile [8, 9]. We are therefore confident that within the power constraints of our study, we were unlikely to miss any clinically relevant effects due to hypothalamic-pituitary-adrenal axis suppression.
We appreciate that measuring short-term effects on surrogate biochemical bone markers such as osteocalcin are unlikely to predict osteoporosis. However a much longer study over several years would be required to evaluate any clinically meaningful effects on bone mineral density.
We cannot exclude the possibility that higher doses than were studied here may have produced adverse effects in the steep part of the dose–response curve for systemic bioactivity. Indeed, MF is licensed to be used up to a maximum dose of 400 µg daily, whereas the maximum recommended dose for TA is 220 µg daily. However, we believe that this would be clinically irrelevant as the doses studied coincided with the plateau of the dose–response curve for clinical efficacy in allergic rhinitis [10, 11].
In summary, using standard prescribed doses over 3 weeks, our results show no detectable systemic bioactivity with TA or MF compared with respective pretreatment washout baseline values, and there were no differences between treatments themselves.
Acknowledgments
This study was supported by an unrestricted educational grant from Aventis.
References
- 1.Lipworth BJ, Seckl JR. Measures for detecting systemic bioactivity with inhaled and intranasal corticosteroids. Thorax. 1997;52:476–482. doi: 10.1136/thx.52.5.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson AM, Sims EJ, McFarlane LC, Lipworth BJ. Effects of intranasal corticosteroids on adrenal, bone, and blood markers of systemic activity in allergic rhinitis. J Allergy Clin Immunol. 1998;102:598–604. doi: 10.1016/s0091-6749(98)70275-1. [DOI] [PubMed] [Google Scholar]
- 3.Affrime MB, Kosoglou T, Thonoor CM, Flannery BE, Herron JM. Mometasone furoate has minimal effects on the hypothalamic-pituitary–adrenal axis when delivered at high doses. Chest. 2000;118:1538–1546. doi: 10.1378/chest.118.6.1538. [DOI] [PubMed] [Google Scholar]
- 4.Lipworth BJ, Jackson CM. Safety of inhaled and intranasal corticosteroids: lessons for the new millennium. Drug Safety. 2000;23:11–33. doi: 10.2165/00002018-200023010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Thorsson L, Dahlstrom K, Edsbacker S, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–161. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derendorf H, Hochhaus G, Rohatagi S, et al. Pharmacokinetics of triamcinolone acetonide after intravenous, oral, and inhaled administration. J Clin Pharmacol. 1995;35:302–305. doi: 10.1002/j.1552-4604.1995.tb04064.x. [DOI] [PubMed] [Google Scholar]
- 7.Argenti D, Shah B, Heald D. A pharmacokinetic study to evaluate the absolute bioavailability of triamcinolone acetonide following inhalation administration. J Clin Pharmacol. 1999;39:695–702. doi: 10.1177/00912709922008335. [DOI] [PubMed] [Google Scholar]
- 8.Wilson AM, Lipworth BJ. 24 hour and fractionated profiles of adrenocortical activity in asthmatic patients receiving inhaled and intranasal corticosteroids. Thorax. 1999;54:20–26. doi: 10.1136/thx.54.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McIntyre HD, Mitchell CA, Bowler SD, Armstrong JG, Wooler JA, Cowley DM. Measuring the systemic effects of inhaled beclomethasone: timed morning urine collections compared with 24 hour specimens. Thorax. 1995;50:1280–1284. doi: 10.1136/thx.50.12.1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bronsky EA, Aaronson DW, Berkowitz RB, et al. Dose ranging study of mometasone furoate (Nasonex) in seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1997;79:51–56. doi: 10.1016/S1081-1206(10)63084-0. [DOI] [PubMed] [Google Scholar]
- 11.Munk ZM, Laforce C, Furst JA, Simpson B, Feiss G, Smith JA. Efficacy and safety of triamcinolone acetonide aqueous nasal spray in patients with seasonal allergic rhinitis. Ann Allergy Asthma Immunol. 1996;77:277–281. doi: 10.1016/S1081-1206(10)63320-0. [DOI] [PubMed] [Google Scholar]


