Abstract
Aims
The goal of this study was to determine the frequencies of CYP1A2* 1C, *1D, *1E and *1F variants in the Egyptian population and compare frequencies with other populations.
Methods
Genotyping was performed in a total of 212 unrelated Egyptian subjects using polymerase chain reaction-restriction fragment length polymorphism assay.
Results
The frequencies of CYP1A2*1C, *1D, *1E and *1F variants in the Egyptian population were 0.07, 0.40, 0.03 and 0.68, respectively. The Egyptians have a lower frequency of CYP1A2*1C, and CYP1A2*1E than the Japanese (0.07 vs 0.21 and 0.03 vs 0.08, respectively), while the frequencies of CYP1A2*1D and CYP1A2*1F did not differ significantly between the two groups. CYP1A2*1F (0.68) frequency in Egyptians was identical to that observed in Caucasians (0.68 among 236 German individuals).
Conclusions
The present study is the first to describe the frequencies of four known allelic variants of CYP1A2 among the Egyptian population. CYP1A2*1C and *1E occurred at frequencies significantly lower than that in Japanese, while similar frequencies were observed for CYP1A2*1D and *1F. The CYP1A2*1F frequency appeared to be identical to that of Caucasians. This does not exclude the possibility of the presence of new mutations relatively specific to the Egyptian population that have not been identified.
Keywords: CYP1A2, Egyptians, pharmacogenetics
Introduction
The cytochrome P450 enzyme CYP1A2 plays a major role in the metabolism of many commonly used drugs, including clozapine, imipramine, caffeine, paracetamol, phenacetin, theophylline, and tacrine [1]. Furthermore, CYP1A2 activates several aromatic amines and thus is a key enzyme in chemical carcinogenesis [2]. Several studies on the CYP1A2-dependent metabolism of caffeine or phenacetin have demonstrated that this enzyme is expressed in human livers at various levels amongst individuals [3, 4], suggesting polymorphic control of enzyme activity. Twelve CYP1A2 variant alleles have been re-ported to date [http:/http://www.imm.ki.se/CYPalleles/cyp1a2.htm]. Among these variants, a G→A mutation at position −2964 in the flanking region of the CYP1A2 gene (CYP1A2*1C) caused a significant decrease in CYP1A2 inducibility measured in terms of the rate of caffeine 3-demethylation in Japanese smokers [5]. Moreover, a C→A mutation in intron 1 at position 734 downstream of the first transcribed nucleotide (CYP1A2*1F) has recently been associated with increased CYP1A2 inducibility [6].
Chida et al. [7] have reported the frequencies of four polymorphisms in the CYP1A2 gene (CYP1A2*1C, *1D, *1E and *1F) amongst the Japanese population. Moreover, Sachse et al. reported that CYP1A2*1F is a common polymorphism and occurs at high frequencies among Caucasians [6], However no information is available on the distribution of CYP1A2 variants amongst the Egyptian population. The aim of the present study was to provide an estimate of the frequencies of CYP1A2*1C, *1D, *1E and *1F variants in a total of 212 Egyptian subjects, laying the foundations for any study potentially related to alterations of CYP1A2 activity among the Egyptian population.
Methods
Subjects
Unrelated Egyptian subjects (n = 212) participated in this study. The Egyptian population is divided into several cultural groups: Bedouins, Nubians, Berbers, Peasants and Urbanites. The subjects who participated in our study were students and staff at Cairo University, thereby considered as Urbanites living in Cairo or other surrounding cities. Each subject gave a sample of about 1 ml of saliva after detailed explanation of the purpose of the study; signed written consent was also obtained from each subject. Genomic DNA was isolated from the saliva using a QIAamp DNA Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer's recommendations. Sample collection and DNA isolation were performed under the supervision and approval of the Dean of the Faculty of Pharmacy of Cairo University. The isolated DNA samples were sent to our laboratory in Japan, and the genotyping protocol was approved by the institutional ethics committees of Tohoku University School of Medicine, Sendai, Japan, and Faculty of Pharmacy, Cairo University, Cairo, Egypt.
Genotyping
Detection of the four CYP1A2 genotypes was performed using a previously described polymerase chain reaction-restriction fragment length polymorphism assay (PCR-RFLP) [7] with minor modifications. The sequences of the PCR primers are given by Chida et al. [7]. PCR amplification consisted of an initial denaturation step at 94°C for 5 min followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s. The final extension step was performed at 72°C for 5 min. All these steps were carried out in an Applied Biosystems (Foster City, CA, USA) thermocycler. CYP1A2*1C, *1D, *1E and *1F were identified by DdeI-, NdeI-, StuI-, and ApaI-restriction digestion, respectively (Figure 1). The amplified DNA fragments were digested and subjected to electrophoresis on a 3% agarose gel.
Figure 1.
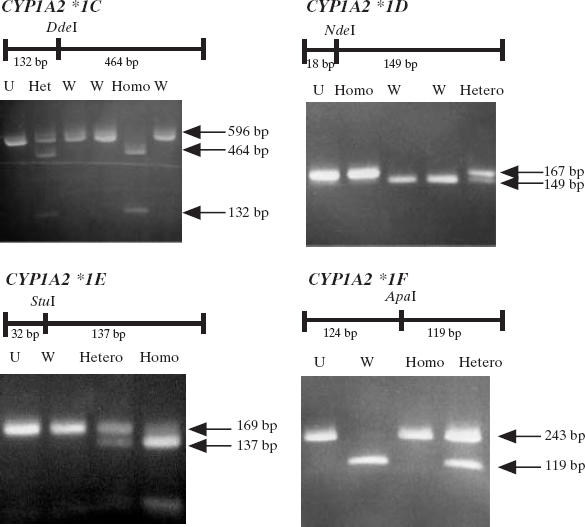
Electrophoresis patterns for CYP1A2 alleles analysed by PCR-RFLP assay. U, Uncut; W, homozygous wild-type; Het, heterozygous mutated allele; Homo, homozygous mutated allele.
Throughout the genotyping assays, positive controls (samples with known genotypes) were not included. Instead, all the subjects were genotyped twice for all the tested mutations, which reduces the possibility of genotyping error.
Statistical analysis
Data were compiled according to the genotype and allele frequencies estimated from the observed numbers for each specific allele. The frequency of each variant is given together with the 95% confidence interval. Differences in allele frequencies between Egyptians and other ethnic populations were measured by Fisher's exact test. A P-value < 0.05 was considered to be statistically significant throughout the population comparisons.
Results
The distribution of the four CYP1A2 genotypes in the Egyptian subjects is summarized in Table 1. The frequencies of the CYP1A2*1C, *1D, *1E and *1F variants were 0.07, 0.40, 0.03 and 0.68, respectively. The 95% confidence intervals for all variants are also given in Table 1. The distribution of the CYP1A2*1C, *1E and *1F genotypes was in accordance with the frequencies expected when applying the Hardy–Weinberg principle (Table 1).
Table 1.
Genotype and allele frequencies of CYP1A2 among Egyptians (present study) and Japanese subjects [7].
| Egyptian (present study) | Japanese [7] | |||
|---|---|---|---|---|
| Polymorphism | Number (n = 212) | Observed frequency | Expected* frequency | Observed frequency (n = 159) |
| CYP1A2*1C | ||||
| G/G | 185 | 87.20 | 86.50 | 61.60 |
| G/A | 25 | 11.80 | 13.00 | 34.60 |
| A/A | 2 | 1.00 | 0.50 | 3.80 |
| G allele vs A variant | 0.93: 0.07 (95% CI, 0.04–0.09) | 0.79: 0.21 (P < 0.0001) | ||
| CYP1A2*1D | ||||
| T/T | 81 | 38.30 | 36.00 | 33.80 |
| T/del | 92 | 43.30 | 48.00 | 48.40 |
| del/del | 39 | 18.40 | 16.00 | 17.80 |
| T allele vs del allele | 0.60: 0.40 (95% CI, 0.35–0.44) | 0.58: 0.42 (N.S.) | ||
| CYP1A2*1E | ||||
| T/T | 199 | 93.80 | 94.10 | 86.20 |
| T/G | 12 | 5.70 | 5.80 | 11.30 |
| G/G | 1 | 0.50 | 0.10 | 2.50 |
| T allele vs A variant | 0.97: 0.03 (95% CI, 0.02–0.05) | 0.92: 0.08 (P = 0.005) | ||
| CYP1A2*1F | ||||
| C/C | 23 | 10.80 | 10.30 | 16.40 |
| C/A | 89 | 42.00 | 43.50 | 44.60 |
| A/A | 100 | 47.20 | 46.20 | 39.00 |
| C allele vs A variant | 0.32: 0.68 (95% CI, 0.64–0.72) | 0.39: 0.61 (N.S.) | ||
n, Total number of subjects.
According to the Hardy–Weinberg law. The 95% confidence interval (95% CI) for all variants in our study is given. Differences in allele frequencies between Egyptians and Japanese were measured by Fisher's exact test. N.S., No significant differences (P > 0.05).
Discussion
Earlier studies using an in vivo caffeine test demonstrated that CYP1A2 activity shows not only interindividual differences (14-fold in the Japanese subjects), but also racial differences in the distribution of probit plots [8]. The racial differences in CYP1A2 activity may be due to exposure to different inducers and/or inhibitors in the diet and environment, and may also reflect different genetic backgrounds [7]. Contrasting the remarkably rich literature on ethnic variation of other CYP isoforms (CYP1A1, CYP2C9, CYP2C19, CYP2D6 and CYP2E1), there are relatively few population studies that have investigated the frequencies of CYP1A2 variants in different ethnic groups. In an early report [9] polymorphisms of other CYP isoforms (CYP2D6 and CYP2E1) were investigated in the Egyptian population. Moreover, in a previous study by our research group [10], we investigated the genotype and allele frequencies of CYP2C9, 2C19 and 2E1 in a total of 247 Egyptian subjects. In the present study, we thought it worthwhile to investigate the prevalence of four polymorphisms in the CYP1A2 gene among 212 Egyptian individuals, providing a basis for future clinical studies regarding variability in CYP1A2 activity and/or inducibility in the Egyptian population.
Preliminary data by Chida et al. [7] suggested that the frequencies of the CYP1A2*1C and *1D variants in Caucasians were lower than those in Japanese, whereas the frequency of the CYP1A2*1F variant was high in Caucasians when compared with Japanese subjects. However, their published data provided no estimates for the actual genotype or allele frequencies of any of the CYP1A2 variants among Caucasians. A comparison between our results and the Japanese study [7] is given in Table 1. The Egyptians have lower frequency of CYP1A2*1C than the Japanese (0.07 vs 0.21) (P < 0.0001); however, the frequency of the CYP1A2*1D allele did not differ significantly between the two groups (Egyptians 0.40, and Japanese 0.42). The Egyptians also had a lower frequency of the CYP1A2*1E allele than the Japanese (0.03 vs 0.08) (P = 0.005).
By contrast, the Egyptians had a higher frequency of the CYP1A2*1F variant (0.68) than the Japanese (0.61); however, statistical analysis showed that there was no significant difference between the two groups (P > 0.05). Recently Sachse et al. [6] reported that the CYP1A2*1F variant exists in Caucasians at a high frequency (0.68 among 236 German individuals). This frequency was identical to that observed in Egyptian subjects in our study (0.68 among 212 Egyptian individuals). This finding may be of clinical importance, as Sachse et al. [6] showed that smokers homozygous for the C-allele had, on average, 40% lower CYP1A2 activity in comparison with those with the A/A genotype. This functional relationship of the C→A polymorphism and CYP1A2 activity could not be found in nonsmokers. Such a genetic variation may have a major influence in altering the response and/or toxicity of a large number of drugs metabolized by CYP1A2, although this needs further investigation.
It is hoped that our results will offer a preliminary basis for more rational use of drugs that are substrates for CYP1A2 in the Egyptian population. It should be mentioned that the present study was designed to investigate the frequencies of four of the known CYP1A1 polymorphisms in the Egyptian population. However, this does not exclude the possibility of the presence of new polymorphisms unique to Egyptians. Further characterization of CYP1A2 genetic polymorphisms among different races might contribute to a better understanding of the molecular basis underlying the large interindividual variation in CYP1A2 activity, and lead to optimization of therapy with a range of clinically important drugs.
References
- 1.Bertz RJ, Grannemann GR. Use of in vitro data and in vivo data to estimate the likelihood of metabolic pharmacokinetic interactions. Clin Pharmacokinet. 1997;32:210–258. doi: 10.2165/00003088-199732030-00004. [DOI] [PubMed] [Google Scholar]
- 2.Coleman T, Ellis SW, Martin IJ, Lennard MS, Tucker GT. 1-Methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) is N-demethylated by cytochromes P450 2D6, 1A2 and 3A4: implications for susceptibility to Parkinson's disease. J Pharmacol Exp Ther. 1996;277:685–690. [PubMed] [Google Scholar]
- 3.Butler MA, Lang MP, Young JF, et al. Determination of CYP1A2 and NAT2 phenotypes in human populations by analysis of caffeine urinary metabolites. Pharmacogenetics. 1992;2:116–127. doi: 10.1097/00008571-199206000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Sesardic D, Boobis AR, Edwards RJ, Davis DS. A form of cytochrome P450 in man, orthologous to form d in the rat, catalyses the O-deethylation of phenacetin and is inducible by cigarette smoking. Br J Clin Pharmacol. 1998;26:363–372. doi: 10.1111/j.1365-2125.1988.tb03393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakajima M, Yokoi T, Mizutani M, Kinoshita M, Funayama M, Kamataki T. Genetic polymorphism in the 5′-flanking region of human CYP1A2 gene: effect on the CYP1A2 inducibility in humans. J Biochem. 1999;125:803–808. doi: 10.1093/oxfordjournals.jbchem.a022352. [DOI] [PubMed] [Google Scholar]
- 6.Sachse C, Brockmöller J, Bauer S, Roots I. Functional significance of the C→A polymorphism in intron I of the cytochrome P450 CYP1A2 gene tested with caffeine. Br J Clin Pharmacol. 1999;47:445–449. doi: 10.1046/j.1365-2125.1999.00898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chida M, Yokoi T, Fukui T, Kinoshita M, Yokota J, Kamataki T. Detection of three genetic polymorphisms in the 5′-flanking region and intron I of human CYP1A2 in the Japanese population. Jpn J Cancer Res. 1999;90:899–902. doi: 10.1111/j.1349-7006.1999.tb00832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima M, Yokoi T, Mizutani M, Shin S, Kadlubar FF, Kamataki T. Phenotyping of CYP1A2 in the Japanese population by analysis of caffeine urinary metabolite: absence of mutation prescribing the phenotypes in the CYP1A2 gene. Cancer Epidemiol Biomarkers Prev. 1994;3:413–421. [PubMed] [Google Scholar]
- 9.Anwar WA, Abdel-Rahman SZ, El-Zein RA, Mostafa HM, Au WW. Genetic polymorphism of GSTM1, CYP2E1 and CYP2D6 in Egyptian bladder cancer patients. Carcinogenesis. 1996;17:1923–1929. doi: 10.1093/carcin/17.9.1923. [DOI] [PubMed] [Google Scholar]
- 10.Hamdy SI, Hiratsuka M, Narahara K, et al. Allele and genotype frequencies of polymorphic cytochromes P450 (CYP2C9, CYP2C19, CYP2E1) and dihydropyrimidine dehydrogenase (DPYD) in the Egyptian Population. Br J Clin Pharmacol. 2002;53:596–603. doi: 10.1046/j.1365-2125.2002.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


