Abstract
Aims
To investigate the distribution of CYP3A activity in the Chinese population, and to test for gender-related differences in CYP3A activity.
Methods
Using midazolam as a probe drug, CYP3A activity in 202 Chinese healthy subjects (104 men) was measured by plasma 1′-hydroxymidazolam:midazolam (1′-OH-MDZ:MDZ) ratio at 1 h after oral administration of 7.5 mg midazolam. The different phases of the menstrual cycle including preovulatory, ovulatory and luteal phases of 66 women phenotyped with midazolam were recorded. The concentrations of 1′-OH-MDZ and MDZ in plasma were measured by HPLC
Results
A 13-fold variation of CYP3A activity (log1′-OH-MDZ:MDZ: range −0.949–0.203) was shown. The CYP3A activity was normally distributed as indicated by the frequency distribution histogram, the probit plot and the Kolmogorov–Smirnov test (P > 0.05). The CYP3A activity of women was higher than that of men (median: −0.36 vs −0.43, P < 0.05; 95% CI for difference: −0.127, −0.012). There was a significant difference in CYP3A activity between the three phases of the menstrual cycle. The activity was highest in the preovulatory phase and decreased sequentially in the ovulatory and luteal phases (P < 0.05).
Conclusions
A normal distribution of CYP3A activity was observed in the Chinese population. The CYP3A activity is higher in female subjects than in males. CYP3A activity differed across the phases of the menstrual cycle.
Keywords: CYP3A, gender, menstrual cycle, midazolam
Introduction
CYP3A is the major drug metabolizing enzyme in the gastrointestinal tract and liver [1]. Four different cDNA clones including CYP3A4, CYP3A5, CYP3A7 and CYP3A43 are classified into the CYP3A subfamily. Overlapping substrate specificities between CYP3A4 and CYP3A5 have previously made it difficult to separate metabolism by these isoforms [2]. Therefore, the term CYP3A in adults is usually understood to reflect the collective activity of CYP3A4 and CYP3A5 [3]. The activity of CYP3A is highly variable, and may influence responses to half of all oxidatively metabolized drugs, as well as endogenous substances such as steroids.
Initial studies suggested that a polymorphism exists in CYP3A4 [5]. Haehner et al. [4] found renal cytochrome CYP3A activity in humans to have a bimodal distribution. This may represent induction of CYP3A5 in a select population and/or a genetically determined organ-specific pattern of expression. However, other studies, while confirming a wide range of interindividual variation in CYP3A4 expression, have failed to confirm bimodality [6]. Wilkinson considered that genetic polymorphism of CYP3A did not appear to exist at least for the liver [1].
Midazolam (MDZ), a typical probe drug of CYP3A, is mainly hydroxylated to 1′-OH-MDZ and 4-hydroxymidazolam (4-OH-MDZ) by CYP3A. In most studies, MDZ clearance (CL) is used to phenotype CYP3A activity but this involves multiple blood sampling over a prolonged interval. In a previous study, we confirmed that there was a significant correlation between plasma MDZ clearance and the 1′-OH-MDZ : MDZ plasma ratio, assessed at 1 h after 7.5 mg MDZ intake in volunteers. The finding provides a simpler estimate for determining liver and intestinal CYP3A activity, using a single blood measurement [7]. Therefore the present study was designed to investigate the distribution characteristics of CYP3A in the Chinese population using the plasma 1′-OH-MDZ : MDZ ratio at 1 h after oral administration of midazolam as a CYP3A activity index.
Some evidence suggests that young women may have approximately 1.4 times the CYP3A4 activity of men [8]. Other reports have not found sex differences in CYP3A4-mediated drug metabolism [8]. Therefore, in this study, we examined possible gender-related differences in CYP3A activity in vivo.
Pregnancy, menopause, oral contraceptive use and menstruation may also have profound effects on drug metabolism, which may be clinically important [8]. In this study, we also investigated whether there was an effect of the menstrual cycle on CYP3A activity by determining the metabolism of midazolam at different phases of the menstrual cycle.
Methods
Subjects
The study protocol was approved by the Ethics Committee of Central South University and all subjects gave written, informed consent before commencing the study.
The study was performed in 104 men and 98 women, who were all Chinese and were judged to be healthy on the basis of medical history, physical examination, and routine clinical laboratory determinations. The men were 20 ± 1 years old (mean ± SD; age range, 18–22 years), and weighed 61 ± 6 kg (weight range, 47–78 kg). Corresponding values for the women were 20 ± 1 years old (mean ± SD; age range, 17–22 years) and 51 ± 5 kg (weight range, 44–65 kg), respectively. The subjects were all nonsmokers and were not regularly taking any medication, including oral contraceptives or other drugs known to inhibit or induce CYP3A. All subjects abstained from ethanol, caffeine, and grapefruit juice for 2 weeks before the study period. The phase of the menstrual cycle of 66 women phenotyped with midazolam was recorded. Three phases of the menstrual cycle were considered: preovulatory (between days 5 and 9), ovulatory (between days 9 and 12) and luteal (between days 20 and 25) [9].
Chemicals and materials
Midazolam and 1’-hydroxymidazolam were purchased from Ultrafine company (Manchester, UK). Nortriptyline was purchased from Sigma Chemical Co (St Louis, USA). Acetonitrile and methanol of HPLC grade and double-distilled water were required for HPLC with u.v. detection. All other chemicals were of AR grade available from commercial sources.
Experimental protocol
After an overnight fast, each subject received 7.5 mg midazolam (Dormicum, Hoffman–La Roche Ltd, Basel, Switzerland) orally with 100 ml water. All subjects continued to fast until the blood sample had been collected. Blood samples (5 ml) were drawn in EDTA tubes at 1 h after drug administration. Harvested plasma was stored at −20 °C until analysis for concentration of MDZ and 1′-OH-MDZ.
HPLC analysis
Midazolam and 1′-hydroxymidazolam concentrations were determined using a method based on that of Carrillo et al. [10]. After adding 100 µl of 100 nmol l−1 nortriptyline as internal standard and 1 ml glycine buffer (0.75 mol l−1, pH 9), 1 ml plasma was extracted with 4 ml diethylether. The organic phase was evaporated to dryness. The residue was dissolved in 50 µl of mobile phase, and 20 µl were injected onto the HPLC column. Midazolam and 1′-hydroxymidazolam were separated on a C8 column (4.6 mm × 150 mm, 5 µm particle size, Hewlett). The composition of the mobile phase was 32% acetonitrile : 3% methanol : 65% 0.1 m buffer acetate (v/v/v) (pH 4.34). The flow rate through the column at 35 °C was 1.1 ml min−1, and midazolam and 1′-hydroxymidazolam were monitored by ultraviolet absorbance at 234 nm. The limit of detection was 8 nmol l−1 for both MDZ and 1′-OH-MDZ. The within- and between-day coefficients of variation were less than 10%.
Data analysis
The data were illustrated by probit plots and frequency distribution histograms and were examined for normality of distribution by the Kolmogorov–Smirnov test of normality. Mean values of CYP3A activity in men and women were compared using Student's t-test. The influence of the menstrual cycle on the metabolism of midazolam among three different phases was estimated by one-way anova. The comparisons of CYP3A activity in each pair of phases were analysed by the least significant difference (LSD) test. These statistical analyses were carried out with SPSS for Windows version 10.0. Values of P < 0.05 were considered significant.
Results
CYP3A activity
The activity (mean ± SD) of CYP3A (log1′-OH-MDZ : MDZ) was −0.43 ± 0.21 (95% CI −0.47, −0.39) in females, −0.36 ± 0.20 (95% CI −0.40, −0.32) in males and −0.39 ± 0.21 (95% CI −0.42, −0.36) in all subjects. A 13-fold variation of CYP3A activity was observed with a range of −0.949–0.203. The CYP3A activities in three phases of the menstrual cycle, assessed by the Kolmogorov–Smirnov test, were all normally distributed (P > 0.05). For female subjects, male subjects and all subjects, the CYP3A activity was shown to be normally distributed as assessed by the frequency distribution histogram (Figure 1), the probit plot (Figure 1+) and the Kolmogorov–Smirnov test (P > 0.05) (Table 1). Therefore it is unlikely that the activity of CYP3A exhibits polymorphism.
Figure 1.
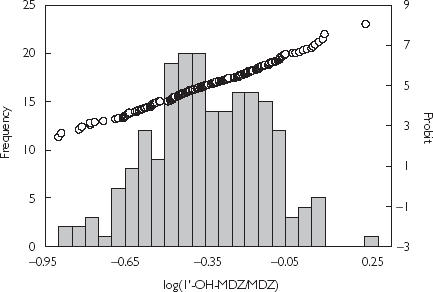
Frequency distribution and and probit plot of CYP3A activity index in 202 Chinese subjects.
Table 1.
CYP3A activity in Chinese subjects and the test of normality for the distribution of CYP3A activity
| Activity (mean ± SD) (log 1′-OH-MDZ : MDZ) | Skewness (g1) | Kolmogorov–Smirnov test SE of skewness (σg1) | Kurtosis (g2) | SE of kurtosis (σg2) | P | |
|---|---|---|---|---|---|---|
| Males (n = 104) | −0.43 ± 0.21 | −0.200 | 0.237 | −0.017 | 0.469 | > 0.05 |
| Females (n = 98) | −0.36 ± 0.20 | 0.081 | 0.244 | −0.569 | 0.483 | > 0.05 |
| Total (n = 202) | −0.39 ± 0.21 | −0.081 | 0.171 | −0.182 | 0.341 | > 0.05 |
Gender-related differences of CYP3A activity
The mean value of log 1′-OH-MDZ : MDZ of women (−0.36) was higher than that of men (−0.43) (P < 0.05) (95% CI of difference: −0.127, −0.012) (Table 2). The probit plot of CYP3A activity in women was shifted to the right compared with men (Figure 2), suggesting a gender difference in CYP3A activity.
Table 2.
Gender difference of CYP3A activity (mean ± SD)
| n | CYP3A activity log 1′-OH-MDZ : MDZ | Mean difference (95% CI) | P value | |
|---|---|---|---|---|
| Male | 104 | −0.43 ± 0.21 | ||
| −0.069 (−0.127, −0.012) | < 0.05 | |||
| Female | 98 | −0.36 ± 0.20 |
Figure 2.
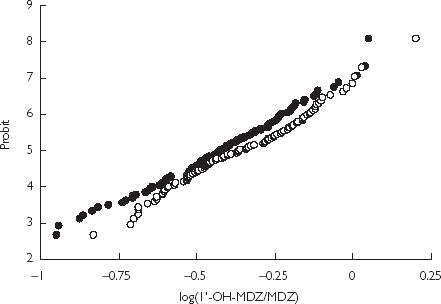
Probit plots of CYP3A activity index in men (•) (n = 104) and and women (○) (n = 98).
The effect of menstrual cycle on CYP3A activity
The activities of CYP3A differ in the three phases of the cycle: preovulatory (−0.28 ± 0.21, 95% CI −0.38, −0.19), ovulatory (−0.39 ± 0.21, 95% CI −0.51, −0.27) and luteal (−0.44 ± 0.19, 95% CI −0.51, −0.37) (Table 3) (n = 66). The 95% CI of the difference of CYP3A activity was −0.043, −0.248 between preovulatory and ovulatory phase, −0.041, −0.267 between preovulatory and luteal phase, and −0.075, −0.177 between ovulatory and luteal phase, respectively.
Table 3.
The activities (mean ± SD) of CYP3A in different phases of menstrual cycle
| P values (Mean difference; 95% CI) | ||||||
|---|---|---|---|---|---|---|
| Phase | n | Activity (log 1′-OH-MDZ : MDZ) | 1 vs 2 | 1 vs 3 | 2 vs 3 | 1 vs 2 vs 3 |
| 1 | 21 | −0.28 ± 0.21 | P > 0.05 | P < 0.05 | P < 0.05 | F = 3.618 |
| 2 | 15 | −0.39 ± 0.21 | (0.102; −0.043, 0.248) | (0.154; −0.041, 0.267) | (0.051; −0.075, 0.177) | P = 0.033 |
| 3 | 30 | −0.44 ± 0.19 | ||||
Phases coded as 1, 2, 3 refer to preovulatory, ovulatory and luteal phase, respectively. The CYP3A activity of phase 1 compared with phase 2 was coded as 1 vs 2. The CYP3A activity of phase 1 compared with phase 3 was coded as 1 vs 3. The CYP3A activity of phase 2 compared with phase 3 was coded as 2 vs 3. The comparison of CYP3A activity in different phases was coded as 1 vs 2 vs 3.
Discussion
Genetic polymorphism is one of the factors resulting in variation of CYP450 activity and gives rise to distinct subgroups in the population that differ in their ability to perform certain drug biotransformation reactions. With respect to the possible polymorphism of CYP3A activity, findings in a number of studies have been inconsistent. Kleinbloesem et al. [5] found that the frequency distribution of the area under the plasma concentration-time curve (AUC) of nifedipine, a CYP3A substrate, was bimodal in a population of 53 healthy subjects. However, in another two studies with 172 and 130 healthy subjects, respectively, bimodality was not observed [6]. In the study in which midazolam was administered intravenously to 168 Caucasian patients, polymorphism in the oxidative metabolism of midazolam was not evident [11]. The metabolism of midazolam reflects hepatic CYP3A activity when administered intravenously, but reflects both intestinal and hepatic CYP3A activity after oral administration [12].
In the present study, we found that the population frequency distribution of the metabolism of midazolam during oral administration in healthy volunteers was unimodal. Therefore, our findings are in accordance with the results of Kassai et al. [11]. Several published reports have described 25 different alleles in CYP3A4 and 12 different alleles in CYP3A5 (http://www.imm.ki.se/cypalleles). Because of the low frequency of these alleles, and the many environmental factors that affect CYP3A activity, the polymorphisms of the CYP3A gene are unlikely to lead to a bimodal distribution of CYP3A phenotype. Genetic polymorphisms may still be important determinants of individual CYP3A activity. In this study, a 13-fold variation of CYP3A activity was observed, which may be due to a combination of genetic, environmental, pathological, hormonal, and dietary factors. Such a remarkable variability of CYP3A activity results in potential difficulties in determining a drug dosage regimen in individual patients whose metabolizing ability is unknown a priori [1]. Some pharmaceutical companies are now screening new chemical entities during the drug development process to determine whether they are metabolized by CYP3A enzymes [13].
Recent advances in the characterization of specific isozymes involved in drug metabolism now allow for the preliminary identification of enzyme systems that are affected by gender. The significance of these gender differences will be most important in the administration of drugs that have a narrow therapeutic range [8]. Erythromycin, whose N-demethylation is mediated exclusively by CYP3A4, is metabolized 25% more rapidly by female than male human liver microsomes [14]. Krecic-Shepard et al. [15] reported that the clearance of nifedipine was significantly lower in men compared with women. Midazolam was found to be cleared 20–40% faster by women than by men, although this did not reach statistical significance [8]. By comparison, there are other reports that have not found gender differences in CYP3A4-mediated drug metabolism. Lobo et al. found that gender did not appear to influence the clearance of nifedipine [16]. Contradictory results have been observed in alfentanil metabolism in relation to gender [17, 18]. Some studies failed to detect a significant gender difference in CYP3A4 concentrations in human liver microsomes and thus do not support the notion of a gender difference in CYP3A4 activity [8].
In this study, using the metabolism of midazolam as an index, we demonstrated that CYP3A activity was significantly higher in women than in men in this Chinese population (P < 0.05). Therefore, many drugs metabolized by CYP3A may be eliminated faster by women. CYP3A plays an important role in the metabolism of several sex steroid hormones including oestrogen, progesterone and testosterone [8, 19, 20], and the mechanism of gender-related difference of CYP3A activity may be due to the fact that steroid hormones may regulate CYP3A activity at the level of gene expression. Hashimoto et al. and Itoh et al. have identified several consensus sequences for transcription factors including three ERE (oestradiol responsive element) and one progesterone/glucocorticoid responsive element (PRE/GRE) in the promoter region of CYP3A4, CYP3A5 and CYP3A7 [21].
The menstrual cycle is a potential source of variability in drug metabolism and disposition in young women and has been a specific focus of recent FDA guidelines, specifically with regard to new drug development [22]. A previous investigation found no difference in the plasma elimination and systemic clearance of the CYP3A4 probe alfentanil between days 2, 13 and 21 of the menstrual cycle, suggesting no menstrual cycle difference in CYP3A4 activity [23]. Kharasch et al. [24] examined CYP3A4 activity during the menstrual cycle using midazolam clearance as the metabolic probe. Midazolam (1 mg i.v.) was administered to 11 female volunteers with normal menstrual cycles on three separate occasions during the same cycles. The results revealed no difference in hepatic CYP3A4 activity on menstrual cycle days 2, 13, and 21. This contrasts with our findings that the metabolism of midazolam was highest in the preovulatory phase group, and the lowest in the luteal phase group. A possible explanation for this discrepancy is the relatively small sample size in the study of Kharasch et al. [24].
Hormonal changes occurring during the menstrual cycle, especially the varying concentrations of progesterone may contribute to differences of CYP3A activity. We hypothesize that high concentrations of steroid hormones could potentially inhibit CYP3A activity. In our previous in vitro study, we found that CYP3A activity could be inhibited by 30% with 50 µm progesterone. However, a concentration of 50 µm is higher than that likely to be seen in vivo. The activity of CYP3A4 and other isozymes could be inactivated by OCs containing steroid hormones [25]. In addition, OCs reduce the metabolism of cyclosporin, a CYP3A substrate [26, 27]. In the study of Lobo et al. in which the gender did not appear to influence the clearance of nifedipine, the result may have been influenced by the fact that one-third of the women in the study were taking OCs [16]. The mechanism of the effect of menstrual cycle on CYP3A activity still needs to be clarified.
In conclusion, CYP3A activity is unimodally distributed and considerable interindividual variability exists. Gender differences in CYP3A activity were observed, which may influence individual susceptibility to adverse effects of particular drugs. Further investigations are required to clarify the mechanisms underlying the gender differences of CYP3A activity and the effect of menstrual cycle on CYP3A activity.
Acknowledgments
This project was supported by China Medical Board 99–697 and 01–755, and the National Nature Science Foundation of China, no. 30000211 and F30130210.
References
- 1.Wilkinson GR. Cytochrome P4503A (CYP3A) metabolism: prediction of in vivo activity in humans. J Pharmacokin Biopharm. 1996;24:475–490. doi: 10.1007/BF02353475. [DOI] [PubMed] [Google Scholar]
- 2.Paulussen A, Lavrijsen K, Bohets H, et al. Two linked mutations in transcriptional regulatory elements of the CYP3A5 gene constitute the major genetic determinant of polymorphic activity in humans. Pharmacogenetics. 2000;10:415–424. doi: 10.1097/00008571-200007000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Thummel KE, Wilkinson GR. In vitro and in vivo drug interactions involving human CYP3A. Ann Rev Pharmacol Toxicol. 1998;38:389–430. doi: 10.1146/annurev.pharmtox.38.1.389. [DOI] [PubMed] [Google Scholar]
- 4.Haehner BD, Gorski JC, Vandenbranden M, et al. Bimodal distribution of renal cytochrome P4503A activity in humans. Mol Pharmacol. 1996;50:52–59. [PubMed] [Google Scholar]
- 5.Kleinbloesem CH, Brummelen PV, Faber H, Danhof M, Vermeulen NPE, Breimer DD. Variability in nifedipine pharmacokinetics and dynamics: a new oxidation polymorphism in man. Biochem Pharmacol. 1984;33:3721–3724. doi: 10.1016/0006-2952(84)90165-5. [DOI] [PubMed] [Google Scholar]
- 6.Schellens JHM, Soons PA, Breimer DD. Lack of bimodality in nifedipine plasma kinetics in a larger population of healthy subjects. Biochem Pharmacol. 1988;37:2507–2510. doi: 10.1016/0006-2952(88)90238-9. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B, Ou-Yang DS, Cheng ZN, Huang SL, Zhou HH. Single plasma sampling to predict oral clearance of the CYP3A probe midazolam. Acta Phamacol Sin. 2001;22:634–638. [PubMed] [Google Scholar]
- 8.Harris RZ, Benet LZ, Schwartz JB. Gender effects in pharmacokinetics and pharmacodynamics. Drugs. 1995;50:222–239. doi: 10.2165/00003495-199550020-00003. [DOI] [PubMed] [Google Scholar]
- 9.Labbé L, Sirois C, Pilote S, et al. Effect of gender, sex hormones, time variables and physiological urinary pH on apparent CYP2D6 activity as assessed by metabolic ratios of marker substrates. Pharmacogenetics. 2000;10:425–438. doi: 10.1097/00008571-200007000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Carrillo JA, Ramos SI, Agundez JAG, Martinez C, Benitez J. Analysis of midazolam and metabolites in plasma by high-performance liquid chromatography: probe of CYP3A. Ther Drug Monit. 1998;20:319–324. doi: 10.1097/00007691-199806000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Kassai A, Toth G, Eichelbaum M, Klotz U. No evidence of a genetic polymorphism in the oxidative metabolism of midazolam. Clin Pharmacokin. 1988;15:319–325. doi: 10.2165/00003088-198815050-00004. [DOI] [PubMed] [Google Scholar]
- 12.Wandel C, Bocker RH, Bohrer H, et al. Relationship between hepatic cytochrome P4503A content and activity and the disposition of midazolam administered orally. Drug Metab Dispos. 1998;26:110–114. [PubMed] [Google Scholar]
- 13.Watkins PB. Noninvasive tests of CYP3A enzymes. Pharmacogenetics. 1994;4:171–184. doi: 10.1097/00008571-199408000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Hunt CM, Westerkam WR, Stave GM. Effect of age and gender on the activity of human hepatic CYP3A. Biochem Pharmacol. 1992;44:275–283. doi: 10.1016/0006-2952(92)90010-g. [DOI] [PubMed] [Google Scholar]
- 15.Krecic-Shepard ME, Park K, Barnas C, Slimko J, Kerwin DR, Schwartz JB. Race and sex influence clearance of nifedipine: results of a population study. Clin Pharmacol Ther. 2000;68:130–142. doi: 10.1067/mcp.2000.108678. [DOI] [PubMed] [Google Scholar]
- 16.Lobo J, Kack DB, Kendall MJ. The intra- and inter-subject variability of nifedipine pharmacokinetics in young volunteers. Eur J Clin Pharmacol. 1986;30:57–60. doi: 10.1007/BF00614196. [DOI] [PubMed] [Google Scholar]
- 17.Lemmens HJM, Burm AGL, Hennis PJ, et al. Influence of age on the pharmacokinetics of alfentanil. Clin Pharmacokinet. 1990;19:416–422. doi: 10.2165/00003088-199019050-00005. [DOI] [PubMed] [Google Scholar]
- 18.Sitar D, Duke PC, Benthuysen JL, et al. Aging and alfentanil disposition in healthy volunteers and surgical patients. Can J Anaesth. 1989;36:149–154. doi: 10.1007/BF03011438. [DOI] [PubMed] [Google Scholar]
- 19.Mäenpää J, Hall SD, Ring HB, Strom SC, Wrighton SA. Human cytochrome P4503A (CYP3A) mediated midazolam metabolism: the effect of assay conditions and regioselective stimulation by α-naphthoflavone, terfenadine and testosterone. Pharmacogenetics. 1998;8:137–155. [PubMed] [Google Scholar]
- 20.Waxman DJ, Attisano C, Guengerich FP, Lapenson DP. Human liver microsomal steroid metabolism: identification of the major microsomal steroid hormone 6β-hydroxylase cytochrome P-450 enzyme. Arch Biochem Biophys. 1988;263:424–436. doi: 10.1016/0003-9861(88)90655-8. [DOI] [PubMed] [Google Scholar]
- 21.Jounaidi Y, Guzelian PS, Maurel P, Vilarem MJ. Sequence of the 5′-flanking region of CYP3A5: comparative analysis with CYP3A4 and CYP3A7. Biochem Res Commun. 1994;205:1741–1747. doi: 10.1006/bbrc.1994.2870. [DOI] [PubMed] [Google Scholar]
- 22.Merkatz RB, Temple R, Sobel S, Feiden K, Kessler DA. Women in clinical trials of new drugs: a change in food and drug administration policy. N Engl J Med. 1993;329:292–296. doi: 10.1056/NEJM199307223290429. [DOI] [PubMed] [Google Scholar]
- 23.Kharasch ED, Russell M, Garton K, Lentz G, Bowdle TA, Cox K. Assessment of cytochrome P450 3A4 activity during the menstrual cycle using alfentanil as a noninvasive probe. Anesthesiology. 1997;87:26–35. doi: 10.1097/00000542-199707000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Kharasch ED, Mautz D, Senn T, Lentz G, Cox K. Menstrual cycle variability in midazolam pharmacokinetics. J Clin Pharmacol. 1999;39:275–280. [PubMed] [Google Scholar]
- 25.Guengerich FP. Inhibition of oral contraceptive steriod – metabolizing enzymes by steroids and drugs. Am J Obstet Gynecol. 1990;163:2159–2163. doi: 10.1016/0002-9378(90)90557-n. [DOI] [PubMed] [Google Scholar]
- 26.Teichmann AT. Influence of oral contraceptives on drug therapy. Am J Obstet Gynecol. 1990;163:2208–2213. doi: 10.1016/0002-9378(90)90563-m. [DOI] [PubMed] [Google Scholar]
- 27.Back DJ, Orme ML. Pharmacokinetic drug interactions with oral contraceptives. Clin Pharmacokinet. 1990;18:472–484. doi: 10.2165/00003088-199018060-00004. [DOI] [PubMed] [Google Scholar]


