Drugs of the nonsteroidal anti-inflammatory drug (NSAID) class can cause perturbations in renal function. NSAID-induced inhibition of renal prostaglandin synthesis may reduce the glomerular filtration rate, thus precipitating retention of electrolytes and water with oedema and in some cases acute renal failure, nephrotic syndrome or papillary necrosis [1]. Experimental and clinical observations suggest that these side-effects are also to be expected with the novel selective cyclooxygenase (COX)-2 inhibitors [1, 2]. This implies that COX-2 inhibitors, like the classical NSAIDs, may be prone to interact with drugs such as lithium [3]. Indeed, in healthy subjects receiving lithium in the presence or absence of celecoxib, the mean maximum serum concentration and area under the time-concentration curve rose by 16 and 18%, respectively, in celecoxib-treated individuals [4]. A paucity of data as to whether this putative interaction may be of clinical significance [5] instigated the present report.
The patient was a 58-year-old woman with a history of musculoskeletal and gastrointestinal complaints and a manic-depressive disorder of many years’ duration. During the previous 2 years, she had been treated with lithium (Li+) 84 mg (12 mmol) in the morning and 126 mg (18 mmol) at night. Regular monitoring of the serum lithium concentrations during this time showed concentrations in the 0.5–0.9 mmol l−1 range. In addition, she used sertraline (100 mg daily), levomepromazine (25 mg at night), esomeprazole (20 mg daily), tibolone (2.5 mg daily) and ibuprofen (400 mg, as needed and up to four times daily).
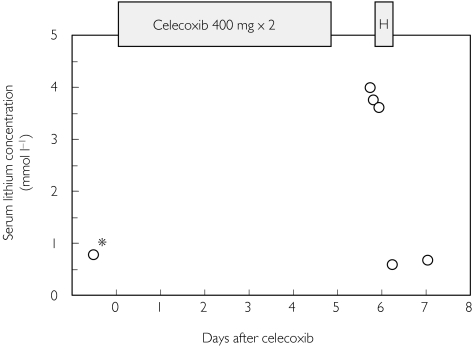
Five days before she was admitted to hospital, she had commenced taking celecoxib 400 mg twice daily. During the following days, she gradually developed a feeling of malaise followed by nausea, abdominal pain, and drowsiness. On admission, she had irregular bradycardia (nadir rate 20 beats min−1) and hypotension. Her serum lithium concentration was 4.0 mmol l−1, and her serum creatinine concentration was 218 µmol l−1. The high lithium concentration was confirmed by repeated measurements the same evening (Figure 1). All drugs were stopped, and she was subsequently treated with haemodialysis for a period of 8 h. Her serum lithium and creatinine concentrations normalized rapidly to precelecoxib levels, with lithium concentrations of 0.57 and 0.67 mmol l−1 and creatinine concentrations of 67 and 90 µmol l−1 on days 2 and 3, respectively (Figure 1). The bradycardia and hypotension resolved during haemodialysis. She was discharged from hospital with sertraline, levomepromazine, esomeprazole, tibolone and ibuprofen as medication. Subsequent follow-ups revealed that the patient had a sinoatrial conduction block. Thus, she has not been rechallenged with the lithium–celecoxib combination.
Figure 1.
Serum lithium concentrations before and after 5 days of celecoxib. *Denotes the last lithium concentration determined prior to the event; H, 8-h haemodialysis treatment.
This chain of events is peculiar in several ways. The patient used two different NSAIDs concomitantly, she took a substantial dose of celecoxib, and she probably had a pre-existing cardiac conduction abnormality. However, she had used lithium with ibuprofen for several years before the addition of celecoxib with no symptoms of toxicity. This is also verified by monitoring of serum lithium concentrations, which were in the therapeutic range on five separate occasions prior to hospitalization. During 5 days of celecoxib 400 mg twice daily, the patient developed serious toxicity concomitant with a five-fold increase in the serum lithium concentration and a close to three-fold increase in the serum creatinine concentration. Careful examination of the patient's history gave no indications that lithium was taken in overdose. In summary, this strongly indicates that the patient was subjected to lithium intoxication due to compromised renal function, and that this was provoked by celecoxib.
There is a previous report in the literature of a 73 year old man who developed side-effects and a moderately high serum lithium concentration of 1. 5 mmol l−1 after 9 days of rofecoxib 12. 5 mg daily [5]. The present case demonstrates that celecoxib may also interact with lithium by raising serum lithium to high and possibly life-threatening concentrations. Caution is warranted when lithium and COX-2 inhibitors are used in combination.
References
- 1.Brater DC. Effects of non-steroidal anti-inflammatory drugs on renal function: focus on cyclooxygenase-2-selective inhibition. Am J Med. 1999;107(6A):65S–71S. doi: 10.1016/s0002-9343(99)00369-1. [DOI] [PubMed] [Google Scholar]
- 2.Whelton A, Schulman G, Wallemark C, et al. Effects of celecoxib and naproxen on renal function in the elderly. Arch Intern Med. 2000;160:1465–1470. doi: 10.1001/archinte.160.10.1465. [DOI] [PubMed] [Google Scholar]
- 3.Garnett WR. Clinical implications of drug interactions with coxibs. Pharmacotherapy. 2001;21:1223–1232. doi: 10.1592/phco.21.15.1223.33891. [DOI] [PubMed] [Google Scholar]
- 4.Celebra. Summary of Product Characteristics. Pharmacia; 1999. 12 03. [Google Scholar]
- 5.Lundmark J, Gunnarson T, Bengtsson F. A possible interaction between lithium and rofecoxib. Br J Clin Pharmacol. 2002;53:403–404. doi: 10.1046/j.1365-2125.2002.01572-1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]