Abstract
Aims
Based on individual case reports of massive overdoses, valproate is often regarded as having significant toxicity. This study aimed to describe the epidemiology of valproate poisoning and the spectrum of its clinical effects.
Methods
Consecutive valproate poisonings were identified and compared with other anticonvulsant overdoses and all other poisonings, from a prospective database of poisoning admissions presenting to a regional toxicology service. National prescription data for the same period were obtained.
Results
There were 79 patients with valproate poisoning from January 1991 to November 2001, 15 cases with valproate alone. Of the 15 cases, drowsiness occurred in two patients (both taking>200 mg kg−1), vomiting occurred in four and tachycardia in five. In patients co-ingesting other medications, moderate to severe effects were consistent with the co-ingestants. There was one death not directly related to valproate. One patient had metabolic acidosis and thrombocytopaenia consistent with severe valproate toxicity. Comparison of valproate, carbamazepine, phenytoin and control groups showed that length of stay for both phenytoin and carbamazepine was significantly longer than for valproate (P < 0.0001), and there was a significantly increased risk of intensive care unit admission for carbamazepine vs valproate (OR 2.73; 95% CI 1.22, 6.28; P = 0.015). Although valproate prescriptions increased over the 10 years, there was relatively greater increase in the incidence of valproate poisoning. The odds of a valproate overdose in 1992 compared with carbamazepine were 0.29 (95% CI 0.07, 1.28; P = 0.141), but in 2001 were 2.73 (95% CI 1.38, 5.39; P = 0.004).
Conclusions
Valproate causes mild toxicity in the majority of cases. Massive overdoses of greater than 400 mg kg−1 can cause severe toxicity, but these are uncommon. The older anticonvulsants phenytoin and carbamazepine remain a greater problem than valproate in overdose.
Keywords: carbamazepine, overdose, poisoning, prescriptions, valproate
Introduction
Sodium valproate or valproic acid is becoming an increasingly common agent to be taken in overdose, with a sharp rise in incidence over the last 5 years [1, 2]. This is likely to be a result of the broadening clinical uses of the drug, including its use as a mood stabilizer in patients with bipolar and affective disorders [3, 4].
Despite valproate being available for over 20 years there are only two large case series of valproate poisoning [1, 5]. However, there is a multitude of case reports of severe toxicity [6–21], giving the impression that valproate has significant toxicity with a narrow therapeutic index. This is not supported in the two previous case series [1, 5] where complications such as coma with or without cerebral oedema, hypotension, pancytopaenia, metabolic acidosis and hyperammonaemia were rare.
We report the only single-centre series of valproate poisonings. The cases occurred over a 10-year period, hence providing information on the epidemiology of valproate poisoning. The relative toxicity of valproate was also investigated using a comparison cohort of other poisoned patients over this 10-year period.
Methods
Hunter Area Toxicology Service database
The Hunter Area Toxicology Service (HATS) is a regional toxicology unit situated at the Newcastle Mater Misericordiae Hospital that services a population of about 350 000 people and is a tertiary referral centre for a further 150 000 [22]. All presentations to emergency departments in the region are either admitted to the unit or notified to HATS and entered prospectively into a clinical database. A preformatted admission sheet is used by medical staff to collect data at the time of admission [23] and this and additional information from the medical record is entered into the database by two trained personnel blinded to any study hypotheses. Detailed demographic and clinical information is recorded [24].
Cohorts studied
The study cases included valproate overdose admissions between January 1991 and November 2001. From this group of valproate overdoses only a patient's first admission was included, all other admissions were excluded. Second and subsequent admissions were excluded to remove the bias of an individual susceptibility to a particular drug.
Three further cohorts of overdose admissions were identified from the HATS database for the same time period, January 1991 to November 2001: a sample of all other poisonings (control group), all phenytoin poisonings and all carbamazepine poisonings. The first group was a control group of poisonings (excluding the valproate group), randomly included from the complete dataset of the HATS database. Three control admissions were included for each of 118 valproate admissions (prior to exclusion of multiple admissions). These were included by randomly taking three admissions from the period 30 days before and after the index valproate admission to give 354 control cases.
Two cohorts were included based on overdose of either phenytoin or carbamazepine. There were 43 phenytoin admissions and 129 carbamazepine admissions. As with valproate, only first admissions were included for analysis. Comparisons were then made between valproate overdoses and each of these other three cohorts of overdoses. In this comparison, patients who co-ingested other drugs were included.
Data analysed
From the database, the following information was obtained: patient demography (sex, age, indication for valproate), details of the valproate ingestion (estimated time of administration, estimation of amount, co-ingestion), clinical features [heart rate (HR), blood pressure (BP) on admission, Glasgow coma score (GCS), gastrointestinal symptoms], investigations [ECG, in-cludng QT, QTc and QRS measurement, biochemistry and haematology tests, valproate concentrations (reference range 350–700 µmol l−1 50–100 mg l−1)], outcomes [mortality, seizures, acidosis, arrhythmias, hypotension, length of stay (LOS) and intensive care unit (ICU) admission], and treatment (decontamination, intravenous rehydration, respiratory and cardiovascular support).
ICU admission criteria for patients presenting to HATS are: ventilated or intubated patients, patients with a decreased level of consciousness (GCS < 9), patients requiring haemodynamic monitoring or circulatory support or have other major organ dysfunction requiring dedicated nursing observation. HATS has a standardized discharge policy requiring review by both the medical toxicology team and the psychiatry team.
Prescription data
Prescription data for Australia were obtained from the Health Insurance Commission for the years 1991–2001 (October). This contains the total number of prescriptions for each drug supplied under the Pharmaceutical Benefits Scheme. The number of prescriptions of all formulations of each of the three anticonvulsants was obtained. Data for 2001 were multiplied by 12/10 to correct for the incomplete numbers of months. The validity of using nationwide statistics for comparisons between drugs taken in the Hunter has previously been established [25]. The numbers of admissions (not just first admissions) presenting with overdoses of valproate, carbamazepine and phenytoin were used for this analysis.
Statistical analysis
For descriptive statistics, means and standard deviations (SD) are quoted for normally distributed data, while medians and interquartile ranges (IQR) are used for nonparametric data. For comparison of two groups, the unpaired t-test and the Mann–Whitney test were used. The Welch correction was used when SDs were significantly different with the unpaired t-test. For continuous variables one-way analysis of variance (anova) was used to compare means of the three groups of anticonvulsants (or four groups when controls were included) when the data was parametric. When continuous variables were not normally distributed or the SDs were significantly different, nonparametric anova (Kruskal–Wallis test) was used. Multiple comparison post tests were used if P < 0.05 with the anova (Tukey for the parametric method and Dunn for the nonparametric method). For comparison of proportions of two groups compared with either control group, Fisher's exact test was used. Using the approximation of Woolf, 95% confidence intervals (95% CI) for odds ratios (OR) were calculated. All statistical analysis was done using GraphPad InStat (version 3.02 for Windows 95; GraphPad Software, San Diego, CA, USA).
Results
Populations
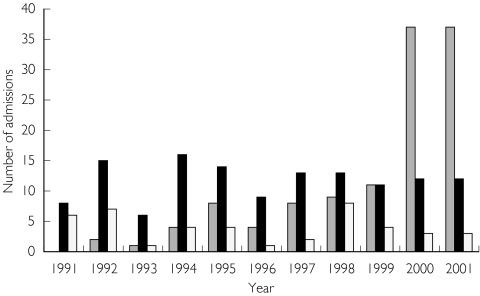
Between January 1991 and November 2001, 126 cases of valproate overdoses presented to HATS. Eight patients were excluded who co-ingested either phenytoin or carbamazepine, leaving 118 cases for analysis. When second and subsequent admissions were excluded, 79 cases remained, and in 15 of these, valproate was the only drug taken in overdose. Fourteen patients presented more than once, up to 11 times for one patient. The patients’ ages ranged from 15 to 66 years, with a median of 31 years (IQR 24–38.5). Valproate was the patient's own medication in 56 cases, ’another person's in eight cases and it was unspecified in the remaining 15. Of the 56 cases, where valproate was the patient’s, in 16 cases it was used as an anticonvulsant, in 38 cases as a mood stabiliser, in 1 case as an adjunct to analgesia, and in another for migraines. Figure 1 shows the distribution of valproate poisoning over the 10-year period illustrating the rapid increase in presentations in the final years of the study.
Figure 1.
The pattern of admission to HATS with overdoses of valproate (118) ( ), phenytoin (43) (▪) and carbamazepine (129)(□) over a 10-year period. These were total numbers of admissions. The data for 2001 is only for 11 months, so each of the three was multiplied by 12/11 to correct for this.
), phenytoin (43) (▪) and carbamazepine (129)(□) over a 10-year period. These were total numbers of admissions. The data for 2001 is only for 11 months, so each of the three was multiplied by 12/11 to correct for this.
Clinical effects of valproate alone
The clinical effects of valproate poisoning in pure ingestions and with co-ingestants are summarized in Table 1. Of the 15 patients ingesting valproate alone, two had drowsiness, both ingesting>200 mg kg−1. Four patients had vomiting and five had tachycardia. No patients had arrhythmias, seizures, coma or hypotension. Only one patient with valproate alone poisoning was admitted to ICU. This patient had no adverse clinical effects and was admitted on the basis of an incorrect dose estimation.
Table 1.
Clinical effects of valproate poisoning in pure ingestions and patients who took other medications in overdose.
| Valproate | ||
|---|---|---|
| Clinical feature | First admissions | Alone |
| Number of cases | 79 | 15 |
| Neurological | ||
| Coma | 2 | 0 (2 drowsy) |
| Seizures | 1 (2)* | 0 |
| Cardiovascular | ||
| Hypotension | 2 | 0 |
| Tachycardia | 25 | 5 |
| Arrhythmia | 0 | 0 |
| Haematological | ||
| Neutropaenia | 1 | 1 |
| Thrombocytopaenia | 4† | 0 |
| Metabolic | ||
| Acidosis | 2 | 0 |
| Hypocalcaemia | 0 | 0 |
| Gastrointestinal | ||
| Minor LFT abnormalities | 1 | 1 |
| Vomiting | 9 | 4 |
One patient had myoclonus, the other had a generalized tonic-clonic seizure.
None of these took co-ingestants with known thrombocytopaenic effects. LFT, liver function test.
Clinical effects of valproate with co-ingestants
There were 64 patients who ingested valproate and other medications in overdose. Only two of the 64 patients had coma, the first co-ingested clonazepam and imipramine, and the second alprazolam and amitriptyline. One patient had a grand mal seizure after taking only 5 g of valproate, but had co-ingested chlorpromazine, risperidone, sertraline and quetiapine. No patients had arrhythmias and only two patients had hypotension. Metabolic acidosis occurred in two patients and no patients had renal failure. There were insufficient ECGs for analysis of the QTc.
Eight patients who took co-ingestants were admitted to ICU. In seven cases the co-ingestants were more likely to be the reason for the admission. One patient who ingested 40 g valproate had severe poisoning with metabolic acidosis and thrombocytopaenia. He was intubated and ventilated and remained in ICU for 5 days. He co-ingested 20 mg pizotifen, which is unlikely to account for these effects. There was one death in a patient who ingested 4 g valproate, but co-ingested diazepam, lithium and glibenclamide and had a 4 h valproate concentra-tion of 768 µmol l−1 (111 mg l−1). She deteriorated and died suddenly. Post-mortem showed acute myocardial ischaemia with atherosclerosis and focal haemorrhage in an atheromatous plaque. It is unlikely that the toxicity of valproate contributed directly to her death.
Dose of valproate
The median dose ingested was 6 g (IQR 3–13.5) for all 79 patients. The median dose for patients who ingested valproate alone was 15 g (IQR 8.25–20), which significantly differed from those who co-ingested another medication, median 5 g (IQR 2.8–10; P = 0.0018). In 8 of the 15 valproate alone poisonings, greater than 200 mg kg−1 (> 14 g) was ingested, none of these had any major abnormalities except drowsiness in two cases ingesting 20 g and 25 g. There was no correlation between dose and severity in all patients with valproate poisoning and in the valproate alone group, using length of stay and ICU admission as indicators of severity.
Valproate concentration
Valproate concentrations were measured in only 48 patients, and there were insufficient samples in most cases to determine accurately peak valproate concentrations. There was no correlation between the measured concentration and severity in this series.
Valproate vs controls and other anticonvulsants
Table 2 shows the baseline characteristics and clinical effects in the valproate overdoses compared with the control group, phenytoin overdoses and carbamazepine overdoses. There were 354 control cases, 103 carbamazepine overdoses and 33 phenytoin overdoses included in the analysis, which were compared with the 79 valproate overdoses. The ages of all four groups were not significantly different, but the gender breakdown was different, with far more females in the valproate group.
Table 2.
Baseline characteristics of valproate, control, phenytoin and carbamazepine poisonings. Median and interquartile ranges (in brackets) are quoted because the data were not normally distributed.
| Valproate | Control | Phenytoin | Carbamazepine | |
|---|---|---|---|---|
| Number | 79 | 354 | 33 | 103 |
| Number of males (%) | 23 (29%)† | 126 (36%) | 15 (55%) | 59 (57%) |
| Age (years) | 31 (24–38.5) | 31 (22–41) | 38 (29–48) | 32 (22–42) |
| Number with co-ingestants (%) | 64 (81%) | – | 23 (70%) | 59 (57%) |
| Time to admission (h) | 2.3 (1.2–4.7) | 2.83 (1.6–6.1) | 5 (1.6–10.3) | 4.16 (1.8–9.6) |
| Length of stay (h) | 18 (11–25) | 17 (9–26) | 27 (17–43)* | 26 (19–54)% |
| Number of ICU admissions (%) | 9 (11%) | 57 (16%) | 7 (21%) | 27 (26%)‡ |
One patient was transgender. significantly different from valproate P < 0.001
Significantly different from valproate P < 0.05
significantly different from valproate, P = 0.0147.
Table 2 also shows a comparison of a number of outcomes for controls, valproate, carbamazepine and phenytoin (first admissions only, including those with co-ingestants). anova of the length of stay of valproate, carbamazepine and phenytoin demonstrated a significant difference between the three (P < 0.0001), with significant differences between valproate and carbamazepine, and valproate and phenytoin. The length of stay for valproate poisonings was not significantly different from controls (P = 0.814). There was no significant difference between the incidence of seizures and arrhythmias for valproate vs carbamazepine and phenytoin. There was a significantly increased risk of ICU admission for carbamazepine vs valproate (OR 2.73, 95% CI 1.22, 6.28, P = 0.0147).
Anticonvulsant prescription data
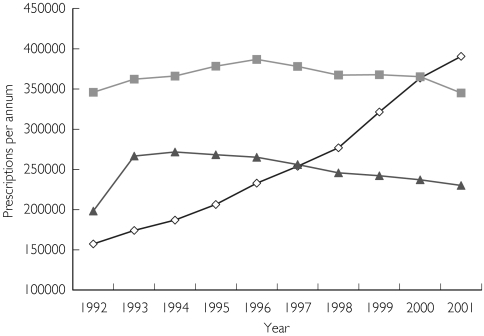
Figure 2 shows the sharp increase in valproate prescriptions during the study period. The rate of poisoning for an individual drug was calculated by adjusting for the number of prescriptions. Using carbamazepine as the comparator the odds ratio of taking an overdose of valproate and phenytoin was calculated at different times to establish whether there had been a change risk of overdose with any one drug. This was calculated for valproate in 1992 and 2001, and phenytoin in 2001. The OR of a valproate overdose in 1992 compared with carbamazepine was 0.29 (95% CI 0.07, 1.28, P = 0.1409), but in 2001 was 2.73 (95% CI 1.38, 5.39, P = 0.0041). The OR for phenytoin overdose in 2001 compared with carbamazepine was 0.41 (95% C.I. 0.11, 1.47, P = 0.2520).
Figure 2.
Figure 2 Prescription data: Graph showing the number of prescriptions per annum (in Australia) for each of the anticonvulsants (⋄, valproate;  , carbamazepine; ▴, phenytoin). There are no easily available data for 1991 and the data for 2001 are only available up to October 2001. Thus, numbers for 2001 were multiplied by 12/10 to correct for this.
, carbamazepine; ▴, phenytoin). There are no easily available data for 1991 and the data for 2001 are only available up to October 2001. Thus, numbers for 2001 were multiplied by 12/10 to correct for this.
Discussion
This is retrospective review of data obtained from a prospective database of consecutive admissions to a single toxicology centre. It describes a series of valproate overdoses and compares valproate poisoning with all other poisonings, and two other important anticonvulsants. The study clearly shows that valproate has a wide toxic index that is not evident from the published case reports.
There have been only two previous large series of valproate overdose [1, 5], the earlier one reported only in French [5]. There have been two smaller series reported in abstract form only; one looking at the changing epidemiology [2] and one at cardiotoxicity and QTc changes [26]. The earliest French series suggested that the majority of valproate poisonings were benign [5] and more recently a US poison centre study described 133 valproate-only overdoses, again demonstrating only mild effects in the majority of cases [1]. This is contrary to the majority of the literature concerning valproate overdose, which consists of numerous case reports of massive overdoses [7, 14, 16–21].
Our study, like the study by Spiller et al. [1], demonstrates that valproate is relatively nontoxic in overdose. There was only one patient in our study who developed any of the features of severe valproate overdose (acidosis and bone marrow suppression) that necessitated admission to ICU. Haemodialysis was not used in any patients. There was one death, but this was not directly related to valproate toxicity. The vast majority of cases had minor effects and short lengths of hospital stay (Table 2).
Our study shows that the common effects of valproate overdose are mild including drowsiness, tachycardia and gastrointestinal effects (mainly vomiting). This is consistent with the two large case series, although one did not report gastrointestinal effects [1, 5, 27]. In larger amounts (> 400 mg kg−1) more severe and life-threatening effects occur [27], including coma, cerebral oedema [28], metabolic acidosis, hyperammonaemia, bone marrow failure (thrombocytopaenia and leukopaenia) and circulatory collapse [1, 5, 27]. These were seen in one patient in this study and have been reported many times in case reports of massive valproate poisoning [6–13, 15].
Bone marrow effects of valproate are well recognized in overdose [1]. In our study there were four patients who had thrombocytopaenia. This may be an underestimation because a full blood count was not taken in all patients. The thrombocytopaenia was delayed in two of the patients in this study. Most of the patients were discharged in under 24 h, so repeat platelet counts after this time may have revealed a higher incidence of mild thrombocytopaenia. The most severe in this series was a minimum platelet count of 63. Spiller et al. demonstrated that thrombocytopaenia occurred with valproate concentrations>450 mg l−1, but did not correlate it with dose ingested [1].
Neutropaenia also occurred in two patients in this study, both also had mild thrombocytopaenia. Again, the incidence of this may have been higher if blood samples were taken 2–5 days after the overdose. Poison centre studies are also likely to underestimate the incidence of mild thrombocytopaenia. It is more likely to be identified in patients admitted with other complications that prolong their length of stay and allow later bloods to be taken. However, there are also likely to be mild cases that are not admitted. These mild cases of thrombocytopaenia are unlikely to have any major problems. A prospective follow-up study would be required to identify the incidence and risk of thrombocytopaenia.
Various treatments have been suggested for valproate poisoning. In this study all patients were managed with supportive care and decontamination with activated charcoal. In severe poisoning [6–13, 15], extracorporeal techniques of drug removal have been employed. One recent case report and review of extracorporeal techniques in valproate poisoning suggests that haemodialysis is more effective than haemoperfusion [15], although this may be limited by the circulatory collapse that can occur. The recent case series of Spiller et al. suggests that haemodialysis should not be used for cases with peak concentrations <850 mg l−1 (5900 µmol l−1) because all patients in that series with concentrations <850 mg l−1 recovered with supportive care [1].
A review of the published case reports reveals a much higher incidence of severe toxicity in valproate poisoning. In all these cases, large amounts of valproate were ingested (when the amount was recorded in the case) or very high concentrations of valproate were measured. This clearly demonstrates the problems with reviewing case reports to determine the incidence and severity of a particular poisoning, in this case valproate. Our study gives a much truer reflection of the spectrum of valproate poisoning seen in a toxicology unit, which is far more useful to emergency medicine physicians and poison centre specialists.
It might be argued that the reason for the less severe clinical effects seen in this case series was due to lower ingested doses overall. This is true, but reflects the reality of the situation. A large number of patients taking valproate in overdose are on multiple psychotropic agents and ingest more than one agent, which has the potential to reduce the total amount of valproate taken. This is consistent with the observation that the valproate-alone overdose had a significantly higher median ingested dose. Because valproate is available as 100, 200 and 500 mg tablets, even if valproate is the only drug taken, many overdoses of a large number of tablets will still not be a large total ingested dose. In large enough amounts, valproate can cause severe toxicity, but in the majority of cases it causes only minor effects.
It has been suggested that overdoses greater than 200 mg kg−1 are potentially toxic [27, 29]. However, in this study more than half of the valproate alone overdoses ingested greater than 200 mg kg−1 with little effect. Drowsiness was seen in two patients ingesting 300–400 mg kg−1, and the only severe poisoning (co-ingesting pizotifen) took>500 mg kg−1. In many previous reports of severe toxicity the dose is not reported [10–13, 15] and where it has been reported, most are over 400 mg kg−1 [6–9]. Unfortunately the more recent case series of Spiller et al. does not report doses or give any indication of severity in relation to ingested dose [1]. It seems that severe toxicity is unlikely to occur with doses less than 400 mg kg−1, and almost never with doses less than 200 mg kg−1.
The majority of patients with valproate overdose can be managed with supportive care and early decontamination with activated charcoal (< 1 h). However, patients ingesting>400 mg kg−1 are more likely to have severe effects and may require early resuscitation and supportive care. Airway protection is essential in patients with coma to allow effective decontamination and prevent aspiration. Circulatory support may be required in the initial stages with fluid resuscitation and then inotropic support if there is persistent hypotension. Patients with significant neurological and cardiovascular effects or with a valproate concentration>850 mg l−1 (5900 µmol l−1) should be considered for haemodialysis. However, severe poisoning is rare and haemodialysis is unlikely to be required in the majority of cases based both our study and the one by Spiller et al. [1].
This study also compares valproate poisoning with poisoning from carbamazepine and phenytoin. The number of patients in this study was not enough to show differences in major outcomes such as coma, arrhythmias and death, but did demonstrate a difference in other measures of severity, such as length of stay in hospital and ICU admission rate. There is significant value in using LOS and ICU admission rate as outcomes for the comparison between valproate, phenytoin and carbamazepine. Although they are only surrogate markers of more severe poisoning and may differ between institutions, the admission policies to both hospital and ICU in this study were the same for all patients. It is thus reasonable to compare between different cohorts of poisonings managed by HATS. However, the absolute LOS and ICU admission rate cannot be directly transferred to other units with differing admission policies. Although there has been an increase in valproate poisonings, carbamazepine poisonings continue to occur and are potentially far more toxic.
Previous investigators have alluded to the increasing incidence of valproate overdose [1, 2], and in our series there was a clear increase in the incidence over 10 years. Figure 1 demonstrates a sharp rise in valproate poisoning in 1999–2001, while Figure 2 shows the corresponding rise in prescription numbers. In addition there is an increased risk of valproate poisoning in 2001 compared with carbamazepine, using number of prescriptions as a surrogate measure of exposure. This is most likely a reflection of the increasing use of valproate in patients with psychiatric disorders.
Valproate has been recognized in the treatment of bipolar disorder and other schizoaffective disorders for over 30 years [4]. In 1994 it was included as a primary agent for bipolar disorder by the American Psychiatry Association in a published practice guideline [4]. The timing would be consistent with a dramatic increase in the use of valproate for psychiatric illness 4–6 years later, as demonstrated in our study and others [1, 2]. Although carbamazepine is also used as a mood-stabilizing agent, its use has been constant over the study period. There was no increased risk of valproate poisoning in 1992, when its major indication was epilepsy [3].
This supports the hypothesis that the increased indications for valproate use, particularly as a mood-stabilizing agent in psychiatric practice, have increased the number of valproate poisonings by making the drug more available to a population with an increased risk of self-harm. The type of substance taken in overdose has previously been shown to be related to its availability [30]. Further, relatively few patients in this study were on valproate for epilepsy, most were on valproate in association with other antipsychotics or with antidepressants. The relatively greater number of carbamazepine overdoses compared with phenytoin also reflects both the broader indications for carbamazepine and that many of these indications are for patients with mood disorders.
A consecutive single-centre series of cases gives a better picture of the spectrum of clinical effects caused by valproate in overdose than do any number of case reports. There has been unnecessary concern about valproate toxicity in the past based on these numerous case reports [6–13, 15]. Here we demonstrate that most cases are benign. However, the case reports do tell us that massive overdoses (> 400 mg kg−1) of valproate are potentially life-threatening and can lead to metabolic acidosis, bone marrow failure, unconsciousness and circulatory collapse. For overdoses greater than 200 mg kg−1 (14 g for 70 kg person) the risk of toxicity is greater, but severe toxicity seems confined to>400 mg kg−1 (30 g for a 70 kg person). It is important for studies of overdose patients that they are collected prospectively, followed in hospital and where possible compared with a control group of poisonings [24].
Acknowledgments
We would like to acknowledge Stuart Allen for extracting the data from the database, and Deb Whyte and Toni Nash for help with retrieving the patient records and entering the data into the database.
References
- 1.Spiller HA, Krenzelok EP, Klein-Schwartz W, et al. Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol. 2000;38:755–760. doi: 10.1081/clt-100102388. [DOI] [PubMed] [Google Scholar]
- 2.Kupferschmidt HHT, Seger DL, Meredith TJ. Changes in indications for valproic acid therapy have led to increased frequency of valproic acid poisoning. J Toxicol Clin Toxicol. 1999;37:411. [] [abstract]. [Google Scholar]
- 3.Lemperiere T. Brief history of the development of valproate in bipolar disorders. Encephale. 2001;27:365–372. [PubMed] [Google Scholar]
- 4.Guay DR. The emerging role of valproate in bipolar disorder and other psychiatric disorders. Pharmacotherapy. 1995;15:631–647. doi: 10.1002/j.1875-9114.1995.tb02874.x. [DOI] [PubMed] [Google Scholar]
- 5.Garnier R, Fournier E. Intoxication aigue par le valproate de sodium. Nouv Presse Med. 1982;11:678. [PubMed] [Google Scholar]
- 6.Van Keulen JG, Van der Deure J, Gemke RJBJ, Van Wijk JAE, Touw DJ. Treatment of valproic acid overdose with continuous arteriovenous hemofiltration. J Toxicol Clin Toxicol. 2000;38:219. [abstract]. [Google Scholar]
- 7.Ishikura H, Matsuo N, Matsubara M, Ishihara T, Takeyama N, Tanaka T. Valproic acid overdose and l-carnitine therapy. J Anal Toxicol. 1996;20:55–58. doi: 10.1093/jat/20.1.55. [DOI] [PubMed] [Google Scholar]
- 8.Graudins A, Aaron CK. Delayed peak serum valproic acid in massive divalproex overdose – treatment with charcoal hemoperfusion. J Toxicol Clin Toxicol. 1996;34:335–341. doi: 10.3109/15563659609013799. [DOI] [PubMed] [Google Scholar]
- 9.Azaroual N, Imbenotte M, Cartigny B, et al. Valproic acid intoxication identified by 1H and 1H-(13) C correlated NMR spectroscopy of urine samples. Magma. 2000;10:177–182. doi: 10.1016/s1352-8661(00)00086-7. [DOI] [PubMed] [Google Scholar]
- 10.Berthelot-Moritz F, Chadda K, Chanavaz I, et al. Fatal sodium valproate poisoning. Intensive Care Med. 1997;23:599. [PubMed] [Google Scholar]
- 11.Brubacher JR, Dahghani P, McKnight D. Delayed toxicity following ingestion of enteric-coated divalproex sodium (Epival) J Emerg Med. 1999;17:463–467. doi: 10.1016/s0736-4679(99)00008-6. [DOI] [PubMed] [Google Scholar]
- 12.Tank JE, Palmer BF. Simultaneous ‘in series’ hemodialysis and hemoperfusion in the management of valproic acid overdose. Am J Kidney Dis. 1993;22:341–344. doi: 10.1016/s0272-6386(12)70329-3. [DOI] [PubMed] [Google Scholar]
- 13.Kane SL, Constantiner M, Staubus AE, Meinecke CD, Sedor JR. High-flux hemodialysis without hemoperfusion is effective in acute valproic acid overdose. Ann Pharmacother. 2000;34:1146–1151. doi: 10.1345/aph.19387. [DOI] [PubMed] [Google Scholar]
- 14.Johnson LZ, Martinez I, Fernandez MC, Davis CP, Kasinath BS. Successful treatment of valproic acid overdose with hemodialysis. Am J Kidney Dis. 1999;33:786–789. doi: 10.1016/s0272-6386(99)70235-0. [DOI] [PubMed] [Google Scholar]
- 15.Hicks LK, McFarlane A. Valproic acid overdose and haemodialysis. Nephrol Dialysis Transplantation. 2001;16:1483–1486. doi: 10.1093/ndt/16.7.1483. [DOI] [PubMed] [Google Scholar]
- 16.Montero FJ. Naloxone in the reversal of coma induced by sodium valproate. Ann Emerg Med. 1999;33:357–358. doi: 10.1016/s0196-0644(99)70381-1. [DOI] [PubMed] [Google Scholar]
- 17.Lee WL, Yang CC, Deng JF, Chen YF, Lin HD, Wang PH. A case of severe hyperammonemia and unconsciousness following sodium valproate intoxication. Vet Human Toxicol. 1998;40:346–348. [PubMed] [Google Scholar]
- 18.Andersen GO, Ritland S. Life threatening intoxication with sodium valproate. J Toxicol Clin Toxicol. 1995;33:279–284. doi: 10.3109/15563659509018000. [DOI] [PubMed] [Google Scholar]
- 19.Connacher AA, Macnab MS, Moody JP, Jung RT. Fatality due to massive overdose of sodium valproate. Scot Med J. 1987;32:85–86. doi: 10.1177/003693308703200312. [DOI] [PubMed] [Google Scholar]
- 20.Khoo SH, Leyland MJ. Cerebral edema following acute sodium valproate overdose. J Toxicol Clin Toxicol. 1992;30:209–214. doi: 10.3109/15563659209038632. [DOI] [PubMed] [Google Scholar]
- 21.Thabet H, Brahmi N, Amamou M, Ben Salah N, Hédhili A. Hyperlactatemia and hyperammonemia as secondary effects of valproic acid poisoning. Am J Emerg Med. 2000;18:508. doi: 10.1053/ajem.2000.3981. [DOI] [PubMed] [Google Scholar]
- 22.Buckley NA, Whyte IM, Dawson AH, McManus PR, Ferguson NW. Self-poisoning in Newcastle, 1987–1992. Med J Aust. 1995;162:190–193. doi: 10.5694/j.1326-5377.1995.tb126020.x. [DOI] [PubMed] [Google Scholar]
- 23.Buckley NA, Whyte IM, Dawson AH, Reith DA. Preformatted admission charts for poisoning admissions facilitate clinical assessment and research. Ann Emerg Med. 1999;34:476–482. [PubMed] [Google Scholar]
- 24.Whyte IM, Buckley NA, Dawson AH. Data collection in clinical toxicology: are there too many variables? J Toxicol Clin Toxicol. 2002;40:223–230. doi: 10.1081/clt-120005492. [DOI] [PubMed] [Google Scholar]
- 25.Buckley N, McManus P. Fatal toxicity of drugs used in the treatment of psychotic illnesses. Br J Psychiatry. 1998;172:461–464. doi: 10.1192/bjp.172.6.461. [DOI] [PubMed] [Google Scholar]
- 26.Kupferschmidt HH, Kevorkian JP, Yang T, et al. Cardiotoxicity in valproic acid poisoning. J Toxicol Clin Toxicol. 1999;37:385. [abstract]. [Google Scholar]
- 27.Jones AL, Proudfoot AT. Features and management of poisoning with modern drugs used to treat epilepsy. Q J Med. 1998;91:325–332. doi: 10.1093/qjmed/91.5.325. [DOI] [PubMed] [Google Scholar]
- 28.Dupuis RE, Lichtman SN, Pollack GM. Acute valproic acid overdose. Clinical course and pharmacokinetic disposition of valproic acid and metabolites. Drug Safety. 1990;5:65–71. doi: 10.2165/00002018-199005010-00006. [DOI] [PubMed] [Google Scholar]
- 29.Garnier R, Boudignat O, Fournier PE. Valproate poisoning. Lancet. 1982;ii:97. doi: 10.1016/s0140-6736(82)91713-5. [DOI] [PubMed] [Google Scholar]
- 30.Hawton K, Ware C, Mistry H, et al. Why patients choose paracetamol for self poisoning and their knowledge of its dangers. Br Med J. 1995;310:164. doi: 10.1136/bmj.310.6973.164. [DOI] [PMC free article] [PubMed] [Google Scholar]