Abstract
Aims
We have previously shown that the systemic exposure to inhaled fluticasone propionate (FP) is reduced in asthmatics compared with healthy subjects. We have now compared its pharmacokinetics in patients suffering from chronic obstructive pulmonary disease (COPD, n = 10) and matched healthy subjects (n = 13).
Methods
A double-blind, randomized, cross-over study design was used. Plasma FP and serum cortisol were measured for 12 h after subjects received hydrofluoroalkane FP 1000 µg day−1 inhaled (via an MDI and spacer) for 7 days and following a single 1000-µg intravenous dose.
Results
The pharmacokinetics differed in the two groups. After inhalation, geometric least square means were significantly lower in the COPD group for the plasma AUC (1961 vs 2996 pg ml−1 h−1 for COPD and controls, respectively; P = 0.03) and the Cmax (235 vs 421 pg ml−1 for COPD and controls, respectively; P = 0.03). Suppression of serum cortisol concentration over 12 h was greater in healthy controls. Weighted mean serum cortisol concentration (nmol l−1) in healthy subjects and COPD was 93 and 170, respectively (P = 0.03). The intravenous pharmacokinetic parameters for FP were comparable in the two groups, resulting in similar suppression of serum cortisol.
Conclusions
We conclude that the altered pharmacokinetics of inhaled fluticasone propionate in COPD caused less hypothalamic–pituitary–adrenal suppression than in healthy controls. This is further evidence that the systemic effects of inhaled corticosteroids should be assessed in patients and not healthy subjects.
Keywords: adrenal, COPD, inhaled corticosteroids, pharmacokinetics
Introduction
Inhaled corticosteroids are widely used to treat chronic obstructive pulmonary disease (COPD). However, the systemic absorption of these drugs can cause side-effects [1–5]. Systemic absorption can occur from two possible routes following inhalation, namely the gut after swallowing, and the lungs. For inhaled fluticasone propionate (FP), oral bioavailability is negligible due both to low absorption from the gastrointestinal tract and high hepatic first-pass metabolism [6]. Therefore, any systemic effects of this drug arise from lung absorption.
We have shown that the systemic availability of FP (1000 µg day−1) is substantially less in patients with asthma compared with healthy controls [7]. This may be due to altered deposition and/or absorption of FP in the lungs of asthmatic patients. The lower systemic availability resulted in reduced hypothalamic–pituitary–adrenal suppression, indicating that conventional inhaled therapeutic doses have less potential for causing systemic side-effects in patients with asthma compared with healthy subjects. Therefore, pharmacokinetics and systemic effects of inhaled corticosteroids and corresponding risk benefit analysis should be studied in patients with the relevant disease rather than in healthy subjects. We now report the first study of the pharmacokinetics and pharmacodynamics of inhaled FP in patients with COPD compared with a matched group of healthy controls.
Methods
Study population
COPD patients aged 42–69 years and healthy volunteers, matched for sex and age, were recruited by means of advertising, with patients being predominantly recruited from the hospital outpatient clinic. We did not use control data from our previous asthma study [7]. All subjects were nonsmokers or ex-smokers for at least 3 months, and were required to refrain from smoking for the duration of the study. COPD was diagnosed using the criteria set out in current guidelines [8] and was based on: (i) a history of cigarette smoking; (ii) symptoms of dyspnoea, cough, sputum production and wheezing; (iii) physical signs (eg hyperinflation, prolonged exhalation); and (iv) airflow obstruction with limited reversibility. Patients were included only if the forced expiratory volume in 1 s (FEV1) was less than 70% of predicted at screening, with less than 15% improvement (or < 200 ml) after inhaled salbutamol (200 µg). Exclusion criteria were: clinically important disease other than COPD, pregnancy or lactation in women, suspected hypersensitivity to inhaled corticosteroids, treatment with oral or parenteral steroids or inhaled FP in the past 6 weeks (inhaled corticosteroids other than FP were allowed), a respiratory tract infection within the past month, bronchiectasis, a previous thoracotomy or the use of regular supplemental oxygen. All subjects gave written informed consent and the study was approved by the South Manchester Local Research Ethics Committee.
Study design
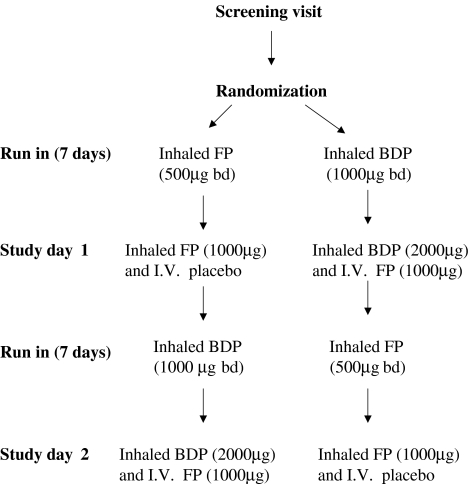
Participants attended an initial screening visit at which pulmonary function tests, including FEV1, FVC, TLC and KCO, and bronchodilator reversibility testing were performed. The inhalers used in this study were hydrofluoroalkane FP (250 µg metered-dose inhaler) and chlorofluorocarbon beclomethasone dipropionate (BDP; 250 µg metered-dose inhaler), and matching placebo inhalers for both FP and BDP. The study was double-blind, and the inhalers and intravenous solutions used could not be distinguished by appearance. Participants were trained initially in an optimum inhalation technique using a spacer device (Volumatic ™; GlaxoWellcome, Greenford, UK) with one dose actuated into the spacer for each inhalation. They were then randomized to receive either one FP and two placebo BDP inhalers, or one placebo FP and two BDP inhalers. Subjects were instructed to take two inhalations from each inhaler at 08.00 h and 20.00 h, for an initial run-in period of at least 7 days (Figure 1). Therefore, the doses during run-in were either 1000 µg FP (500 µg twice daily), or a therapeutically equivalent dose of CFC BDP (1000 µg twice daily). During the run-in periods, adherence was assessed by weighing the aerosol canisters before and after each treatment period. On study day 1, participants received either inhaled FP after FP run-in, or intravenous FP after BDP run-in. Subjects then crossed over into the second run-in and received study day 2 treatments. BDP was used to provide similar effects to FP before subjects received intravenous FP. Inhaled FP could not be used for this purpose as it would have interfered with the pharmacokinetic analysis of the intravenous drug.
Figure 1.
Study design. FP, Fluticasone propionate; BDP, beclomethasone dipropionate.
Prior to each study day, participants were required to fast overnight and refrain from alcohol for 24 h. At 07.00 h on each study day, subjects performed a baseline spirometry measurement, and then ate a standard breakfast. At 08.00 h the subjects were given either 1000 µg inhaled FP and intravenous placebo (0.9% sodium chloride), or inhaled placebo and 1000 µg intravenous FP (in 2 ml of propylene glycol). Inhalations were performed in a sitting position. Infusions were administered into a forearm venous catheter over 10 min using a syringe driver. Venous blood samples were taken from a second venous catheter in the contralateral forearm at predose and at 10 min, 20 min, 30 min, 45 min and 1, 2, 3, 4, 6, 8 and 12 h after dosing to measure plasma concentrations of FP. Blood samples were also taken at predose and 1, 2, 3, 4, 6, 8 and 12 h after dosing for the measurement of serum cortisol concentrations.
In addition to establishing serum cortisol profiles on the study days, serum cortisol concentrations at 08.00 h at the screening visit, and 24 h urinary cortisol excretion at the screening visit and over the 24-h periods prior to each of the two study days were measured.
Measurements
All pulmonary function tests were performed using a VMAX22 spirometer (Sensor Medics BV, Bilthoven, The Netherlands) and a plethysmograph (Autobox 6200 DL; Sensor Medics BV).
Blood samples were drawn into heparinized tubes for plasma FP assays and into plain plastic tubes for serum cortisol assays. These were immediately placed on ice and centrifuged within 30 min at 600 g for 10 min at 4°C. Plasma, serum and urine samples were immediately frozen at −70°C until assay. Blinded analysis of these samples was performed at GlaxoWellcome Research and Development (Ware, UK). Fluticasone propionate was isolated from plasma by solid-phase extraction liquid chromatography-tandem mass spectrometry (LC-MS-MS) that uses thermally and pneumatically assisted electrospray ionization. The assay has a lower limit of detection of 10 pg ml−1, with between- and within-assay coefficients of variation of < 6%. Serum and urinary cortisol concentrations were measured by a competitive immunoassay with use of direct chemiluminescence (Product 672303 and ACS:180SE; Chiron Diagnostics, Harefield, UK). For this assay the lower limit of detection is 6 nmol l−1 and the coefficient of variation is < 3%.
Statistical analysis
The sample size was calculated based on the primary outcome variable, namely the area under the curve (AUC) for plasma FP concentrations. Within-subject variability was estimated using the results of a previous study with a similar design [7]. To detect a significant difference of 40% with a statistical power of 80%, it was estimated that 13 participants in each group would be required. Data were analysed by intention to treat. Pharmacokinetic data were analysed by a conventional noncompartmental approach with WinNonLin Pro software (version 3.0). Systemic bioavailability was calculated, with reference to the nominal dose, as the area under the concentration–time curve after inhalation divided by the AUC after intravenous administration. Plasma clearance and apparent volume of distribution at steady state were calculated by conventional equations. Twenty-four hour urinary cortisol concentrations were multiplied by the 24-h urine volume to convert to the amount of total free cortisol excreted in 24 h. Pharmacokinetic and cortisol data were log-normalized for group comparisons and are presented as geometric least square means with 95% confidence intervals. The area under the serum cortisol–time curve over 12 h was calculated using the trapezoidal method, and the weighted mean serum cortisol concentration obtained by dividing the AUC by the time interval. Mixed effects analyses of variance, with subject as a random effect, were used to compare treatments, populations and any interactions between these factors. Significance levels were set at 0.05 for each contrast. Statistical analyses were performed with SAS version 6.12 software, running on a Unix platform.
Results
Twenty-one COPD patients and 13 healthy subjects were consented and screened for the study. All of the healthy subjects and 11 of the COPD patients were suitable and therefore randomized. One patient was withdrawn after randomization and prior to study day 1 due to a chest infection, so 10 patients completed the study. The baseline characteristics (age and sex) of the patients and healthy controls were similar (Table 1). Eight of the COPD patients were taking inhaled corticosteroids at screening. The baseline measurements of lung function in the patient group were compatible with moderate to severe COPD (FEV1 41% predicted, raised TLC and decreased KCO).
Table 1.
Baseline characteristics of the subjects.
| Characteristics | Controls (n = 13) | COPD group (n = 10) |
|---|---|---|
| Demography | ||
| Age (years) | 59 (5) | 60 (8) |
| Sex (M/F) | 8/5 | 7/3 |
| Weight (kg) | 75.4 (12.3) | 76.2 (19.0) |
| Spirometry | ||
| FEV1 (l) | 3.12 (0.66) | 1.03 (0.39) |
| FEV1 (% predicted) | 109 (15) | 41 (17) |
| FVC (l) | 3.97 (0.71) | 2.17 (0.87) |
| FEV1/FVC (%) | 79 (5) | 42 (15) |
| TLC (l) | 5.6 (1.44) | 7.48 (3.21) |
| TLC (% predicted) | 93 (15) | 125 (30) |
| KCO | 1.49 (0.35) | 0.83 (0.33) |
| KCO (% predicted) | 104 (25) | 57 (24) |
| Daily inhaled steroid dose (µg)* | 900 (0–2000) | |
| Smoking history | ||
| No of pack years* | 0 (0–10) | 37.5 (15–140) |
Data are mean (s.d.), except for
which denotes median (range). Inhaled steroid dose is the dose of beclomethasone diproprionate or budesonide at entry into the study.
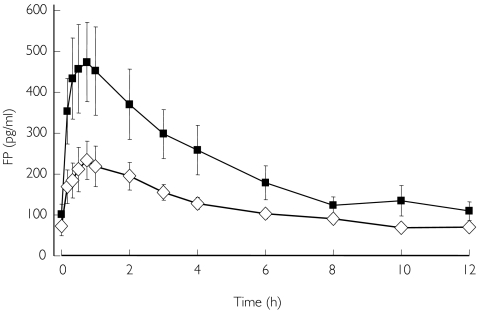
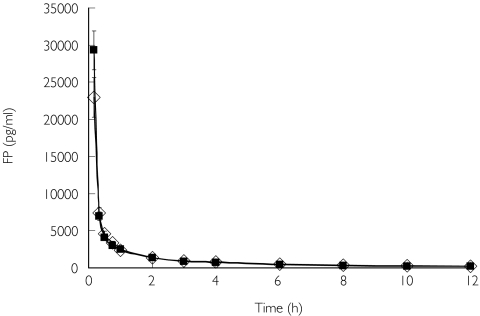
The pharmacokinetics of inhaled FP differed between the two groups (Table 2, Figure 2). There was a significantly lower plasma AUC and Cmax in the COPD patients. The systemic availability of inhaled FP was reduced in COPD but did not reach the level of statistical significance. Intravenous pharmacokinetic parameters for FP were similar in the two groups (Figure 3).
Table 2.
Inhaled and intravenous pharmacokinetic parameters.
| Controls (n = 13) | COPD group (n = 10) | P-value | Ratio COPD/healthy (90% CI) | |
|---|---|---|---|---|
| Pharmacokinetic data inhaled route | ||||
| Area under curve (pg ml−1 h) | 2996 (2337–3840) | 1961 (1478–2602) | 0.03 | 0.65 (0.48–0.89) |
| Cmax (pg ml−1) | 421 (298–594) | 235 (159–348) | 0.03 | 0.56 (0.36–0.86) |
| Systemic availability (%) | 21.2 (14.3–31.4) | 13.3 (8.5–20.9) | 0.12 | 0.63 (0.38–1.03) |
| Tmax (h)* | 0.75 (0.333–3.0) | 0.75 (0.167–3.0) | ||
| Plasma half-life (h) | 6.6 (5.4–8.1) | 7.2 (5.7–9.1) | 0.57 | 1.09 (0.84–1.41) |
| Mean residence time (h) | 9.07 (7.58–10.85) | 10.30 (8.39–12.64) | 0.34 | 1.14 (0.91–1.44) |
| Mean absorption time (h) | 6.19 (4.57–8.37) | 5.51 (3.96–7.67) | 0.60 | 0.89 (0.61–1.29) |
| Pharmacokinetic data intravenous route | ||||
| Area under curve (pg ml−1 h) | 14148 (11038–18135) | 14710 (11086–19518) | 0.83 | 1.04 (0.76–1.42) |
| Cmax (pg/ml) | 23364 (16772–32545) | 18926 (12973–27611) | 0.39 | 0.81 (0.53–1.23) |
| Volume of distribution at steady state (l) | 229 (180–292) | 273 (207–360) | 0.33 | 1.19 (0.88–1.62) |
| Plasma half-life (h) | 4.1 (3.3–5.0) | 4.9 (3.9–6.2) | 0.21 | 1.21 (0.94–1.56) |
| Mean residence time (h) | 3.26 (2.61–4.06) | 4.02 (3.13–5.17) | 0.20 | 1.23 (0.94–1.63) |
| Clearance (ml min−1) | 1173 (1044–1319) | 1133 (992–1295) | 0.69 | 0.97 (0.83–1.12) |
Data are geometric least square means (95% confidence intervals), except for
Inhaled time to Cmax, which is median (range).
Figure 2.
Plasma fluticasone propionate (FP) concentrations after inhalation of 1000 µg. Group mean (s.e.) shown. ⋄, COPD; ▪, controls.
Figure 3.
Plasma fluticasone propionate (FP) concentrations after intravenous administration of 1000 µg. Group mean (s.e.) shown. ⋄, COPD; ▪, controls.
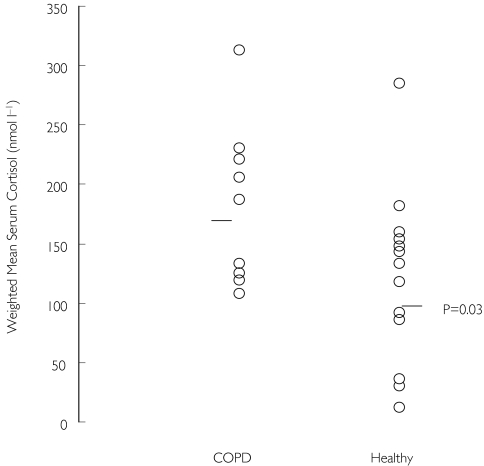
There were no differences at screening between COPD patients and healthy controls in either the morning serum cortisol or 24-h urinary cortisol excretion measurements. Seven days treatment with inhaled FP (1000 µg day−1) did not cause suppression of morning serum cortisol concentrations in either group, although the measurements were lower in healthy subjects compared with COPD. A further single dose of inhaled FP (1000 µg) caused greater suppression of serum cortisol over 12 h in healthy controls compared with COPD patients (individual data shown in Figure 4). However, a single dose of intravenous FP (1000 µg) caused similar suppression of serum cortisol over 12 h in the two groups.
Figure 4.
Weighted mean serum cortisol concentrations over 12 h immediately after inhalation of 1000 µg fluticasone propionate (after fluticasone propionate 1000 µg day−1 for 7 days). Only nine samples were obtained from the COPD group. s denotes median value, P-value is between-group comparison.
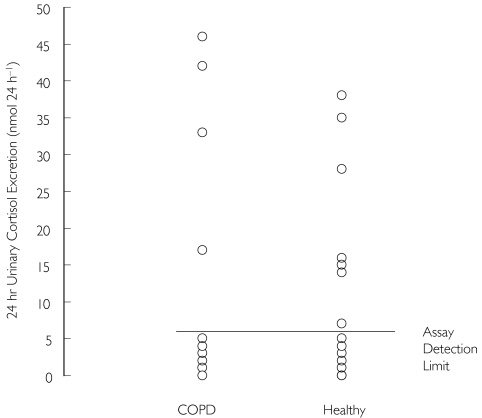
Seven days’ treatment with either inhaled FP (1000 µg day−1) or BDP (2000 µg day−1) caused a reduction in 24-h urinary cortisol excretion in both COPD patients and healthy controls, with most values being below the limit of quantification of the assay (individual data after inhaled FP are shown in Figure 5).
Figure 5.
24-h urinary cortisol excretion after 7 days’ treatment with inhaled fluticasone propionate (1000 µg day−1)
Discussion
This study has demonstrated that the pharmacokinetics of inhaled FP are altered in COPD compared with healthy controls, with the Cmax and AUC being substantially lower in COPD. Consequently, using serial measurements of serum cortisol over 12 h to measure hypothalamic–pituitary–adrenal suppression, there were less systemic effects in the COPD group. These findings are similar to those from our previous comparison of the pharmacokinetics and pharmacodynamics of inhaled FP in moderate/severe asthma [7]. The absorption and systemic effects of this inhaled corticosteroid therefore appear to be reduced in patients with moderate to severe airflow obstruction (irrespective of the diagnostic label of asthma or COPD) compared with healthy controls.
We found a significant reduction in the systemic exposure to inhaled FP (Cmax and AUC) in COPD, suggesting decreased systemic absorption in COPD. The difference in absolute bioavailability for FP in COPD and healthy subjects, although similar to that found for AUC and Cmax, did not reach the level of statistical significance due to the variability and small number of subjects in this study. We concluded that these pharmacokinetic differences were caused by alterations in absorption and/or deposition from the lung, as there were no differences between the two groups after the administration of intravenous FP.
We found that there was a numerical difference (that was not statistically significant) in the morning (08.00 h) cortisol concentrations between the two groups at screening, with the COPD values being greater despite the majority taking inhaled steroids at screening. However, these single cortisol measurements should be interpreted with caution, as serum cortisol concentrations are known to vary greatly over even a few minutes [9]. Consequently, 24-h urine cortisol collection and serial serum cortisol measurements are more sensitive and reliable methods of assessing adrenal function [9, 10]. We found that the 24-h urine cortisol concentrations at screening were numerically lower in the COPD patients, which would be expected in subjects treated with inhaled corticosteroids, although this difference was not statistically significant. Taken together, these serum and urine cortisol results indicate that adrenal function in the two groups at screening was similar. After 1 week of inhaled FP (1000 µg day−1) the morning cortisol concentrations were not suppressed in either healthy subjects or COPD patients, although again these single cortisol measurements need to be interpreted with caution. Serial serum cortisol measurements over 12 h after a further dose of inhaled FP (1000 µg) produced greater suppression of serum cortisol over 12 h in healthy subjects compared with COPD patients. However, intravenous FP (1000 µg) caused a similar reduction in serum cortisol over 12 h in the two groups. These results indicate that inhaled FP produces diminished systemic effects in COPD due to reduced pulmonary absorption in COPD.
The 24-h urinary excretion of free cortisol is normally very low, as most is bound to proteins in blood and normal values can be close to the assay detection limit. At these low concentrations, there can be a decrease in the sensitivity of the assay, so that the values obtained do not accurately reflect the circulating cortisol concentration or cortisol production rate [9]. We found that there was a similar reduction in 24-h urinary cortisol excretion in healthy subjects and COPD patients after 1 week of treatment with 1000 µg day−1 inhaled FP. This was because the majority of the results were below the limit of detection of the assay. However, we were able to assess differences in adrenal function between the two groups by measuring serial serum cortisol over 12 h, which is a sensitive method for evaluating changes in cortisol production [9, 10].
For many years, the results of studies performed in healthy volunteers have been used to make judgements about the risk-benefit of inhaled corticosteroids in COPD [11–14]. However, subjects with COPD have airflow obstruction, and often have airway inflammation. These pathological and physiological changes cause altered particle deposition in the lung after inhalation [15]. It has been shown in asthma that the extent of suppression of serum cortisol after a 500-µg dose of inhaled FP is related to the FEV1, suggesting that the degree of airflow obstruction is the key determinant of the systemic activity of FP [10]. The current study has demonstrated that altered deposition, and/or altered absorption, of inhaled FP in COPD patients results in reduced systemic effects, and underscores the inappropriateness of studies of fluticasone in healthy subjects.
Harrison et al. [16] demonstrated that inhaled FP had a greater effect on urinary total cortisol metabolite concentrations in healthy subjects compared with patients with asthma, although this was not the case with budesonide. This suggests that the difference in systemic effects seen between healthy subjects and patients with airflow obstruction can vary with the inhaled corticosteroid studied. This may be due to differences in device performance, such as the particle size range in the delivered dose and the degree of central vs peripheral lung deposition, and/or differences in the gastrointestinal and lung absorption characteristics between different inhaled corticosteroids. In particular, the degree of mucociliary clearance may be an important determinant of corticosteroid absorption from the lungs in COPD. Therefore, systemic effects of other inhaled corticosteroids in COPD need to be studied, as they may be different from those of inhaled FP described in this paper.
The recruitment of patients for this study did not specify criteria for lung function apart from FEV1, as we did not set out to select a subgroup of COPD patients. However, by chance our patients had reduced KCO measurements and raised TLC, indicating predominant emphysema. Our study proves the hypothesis that fluticasone pharmacokinetics and systemic effects are altered in COPD, and we suggest that future studies investigate whether there are differences between COPD patients with and without emphysema.
The systemic effects of inhaled corticosteroids may cause complications such as cataracts [2], loss of glycaemic control [3], skin bruising [4] and osteoporosis [5]. However, a study in patients with moderate to severe COPD has shown a relatively low rate of skin bruising (7%), bone fractures (2%) and cataracts (1%) following treatment with FP 1000 µg daily for 3 years [1]. Further studies are needed to define the doses of inhaled corticosteroids which cause these effects in COPD, as reduced pulmonary absorption of inhaled corticosteroids in COPD may allow higher doses to be safely administered in this patient group, especially during exacerbations of disease. In addition, other studies are needed to investigate the reasons for reduced pulmonary absorption in COPD.
Table 3.
Cortisol measurements.
| Controls (n = 13) | COPD (n = 10) | P-value | Ratio COPD/healthy (95% CI) | |
|---|---|---|---|---|
| Serum morning cortisol concentration (nmol l−1) | ||||
| At screening | 334 (262–425) | 457 (342–611) | 0.10 | 1.37 (1.00–1.88) |
| After 7 days fluticasone propionate (500 µg BD) | 352 (274–452) | 545 (408–728) | 0.03 | 1.55 (1.12–2.13) |
| After 7 days BDP (1000 µg BD) | 368 (289–469) | 560 (405–774) | 0.04 | 1.52 (1.09–2.13) |
| Weighted mean serum cortisol over 12 h (nmol l−1) | ||||
| After 1000 µg inhaled fluticasone propionate | 93 (66–131) | 170 (113–258) | 0.03 | 1.83 (1.07–3.13) |
| After 1000 µg intravenous fluticasone propionate | 89 (63–126) | 124 (82–127) | 0.22 | 1.38 (0.81–2.37) |
| 24-h urinary cortisol excretion (nmol per 24 h)* | ||||
| At screening | 56 (39–80) | 37 (24–56) | 0.13 | 0.66 (0.38–1.14) |
| After 7 days fluticasone propionate (500 µg BD) | 0 (0–115) | 0 (0–71) | ||
| After 7 days BDP (1000 µg BD) | 0 (0–59) | 7 (0–54) | ||
Data are geometric least square means (95% confidence interval) except for
which is median (range). For 24-h urinary cortisol excretion, values below the assay detection limit of 6 nmol per 24 h were recorded as zero. BD, Twice daily.
Acknowledgments
This study was supported by GlaxoSmithKline. We are grateful to Carole Bye for conducting the statistical analysis and Sheryl Callejas for the analysis of fluticasone propionate.
References
- 1.Burge P, Calverley P, Jones P, Spencer S, Anderson J, Maslen T. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cumming RG, Mitchel P, Leeder S. Use of inhaled corticosteroids and the risk of cataracts. N Engl J Med. 1997;337:8–14. doi: 10.1056/NEJM199707033370102. [DOI] [PubMed] [Google Scholar]
- 3.Faul JL, Tormey W, Burke C. High dose inhaled corticosteroids and dose dependant loss of diabetic control. BMJ. 1999;317:1491. doi: 10.1136/bmj.317.7171.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pauwels RA, Lofdal CG, Laitinen LA, et al. Long term treatment with inhaled budesonide in persons with mild chronic obstructive pulmonary disease who continue to smoke. N Engl J Med. 1999;340:1948–1953. doi: 10.1056/NEJM199906243402503. [DOI] [PubMed] [Google Scholar]
- 5.Wong CA, Walsh LJ, Smith CJP, et al. Inhaled corticosteroids use and bone-mineral density in patients with asthma. Lancet. 2000;355:1399–1403. doi: 10.1016/S0140-6736(00)02138-3. [DOI] [PubMed] [Google Scholar]
- 6.Falcoz C, Oliver R, McDowall J, Ventresca P, Bye A, Daley-Yates P. Bioavailability of orally administered micronised fluticasone propionate. Clin Pharmacokinet. 2000;39(Suppl. 1):9–15. doi: 10.2165/00003088-200039001-00002. [DOI] [PubMed] [Google Scholar]
- 7.Brutsche MH, Carlen Brutsche I, Munawer M, et al. Comparison of pharmacokinetics and systemic effects of inhaled fluticasone propionate in patients with asthma and healthy volunteers: a randomised crossover study. Lancet. 2000;356:556–561. doi: 10.1016/S0140-6736(00)02581-2. [DOI] [PubMed] [Google Scholar]
- 8.The COPD Guideline Group of the Standards of Care Committee of the BTS. BTS guidelines for the management of chronic obstructive pulmonary disease. Thorax. 1997;52(Suppl. 5):S1–S28. [PMC free article] [PubMed] [Google Scholar]
- 9.Dekhuijzen PN, Honour JW. Inhaled corticosteroids and the hypothalamic–pituitary–adrenal (HPA) axis: do we understand their interaction ? Respir Med. 2000;94:627–631. doi: 10.1053/rmed.2000.0791. [DOI] [PubMed] [Google Scholar]
- 10.Weiner P, Berar-Yaney N, Davidovich A, Magadle R. Nocturnal cortisol secretion in asthmatic patients after inhalation of fluticasone propionate. Chest. 1999;116:931–934. doi: 10.1378/chest.116.4.931. [DOI] [PubMed] [Google Scholar]
- 11.Grahnen A, Eckernas SA, Brundin RM, Ling-Andersson A. An assessment of the systemic activity of single doses of inhaled fluticasone propionate in healthy volunteers. Br J Clin Pharmacol. 1994;38:521–525. doi: 10.1111/j.1365-2125.1994.tb04393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grahnen A, Jansson B, Brundin RM, et al. A dose–response study comparing suppression of plasma cortisol induced by fluticasone propionate from Diskhaler and budesonide from Turbohaler. Eur J Clin Pharmacol. 1997;52:261–267. doi: 10.1007/s002280050287. [DOI] [PubMed] [Google Scholar]
- 13.Lonnebo A, Grahnen A, Jansson B, Brundin RM, Ling-Andersson A, Eckernas SA. Assessment of the systemic effects of single and repeated doses of inhaled fluticasone propionate and inhaled budesonide in healthy volunteers. Eur J Clin Pharmacol. 1996;49:459–463. doi: 10.1007/BF00195931. [DOI] [PubMed] [Google Scholar]
- 14.Thorssom L, Dahlstrom K, Edsbacker A, Kallen A, Paulson J, Wiren JE. Pharmacokinetics and systemic effects of inhaled fluticasone propionate in healthy subjects. Br J Clin Pharmacol. 1997;43:155–161. doi: 10.1046/j.1365-2125.1997.d01-1425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yanai M, Hatazawa J, Ojima F, Sasaki H, Itoh M, Ido T. Deposition and clearance of inhaled 18FDG powder in patients with chronic obstructive pulmonary disease. Eur Respir J. 1998;11:1342–1348. doi: 10.1183/09031936.98.11061342. [DOI] [PubMed] [Google Scholar]
- 16.Harrison TW, Wiseiewski A, Honour J, Tattersfield AE. Comparison of the systemic effects of fluticasone propionate and budesonide given by dry powder inhaler in healthy and asthmatic subjects. Thorax. 2001;56:186–191. doi: 10.1136/thorax.56.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]