Abstract
Aims
The aim of this study was to clarify the pharmacokinetics of ribavirin and interferon-alpha (IFN-α) 2b when administered in combination for 24 weeks and effects of pharmacokinetics of both on treatment outcome in chronic hepatitis C with genotype 1b and high viral load.
Methods
In this multicentre open study, 27 patients received 2-week daily induction therapy followed by 22-week, three-times-a-week maintenance therapy of intramuscular IFN-α 2b at a dose of 6 million units and oral ribavirin at 400 mg twice daily for 24 weeks, and followed up for 24 weeks post-treatment. Single- and multiple-dose pharmacokinetic studies were assessed by serial measurements of serum concentrations of both compounds at weeks 1 and 24.
Results
Five patients attained sustained virological response. Serum ribavirin concentrations asymptoted after 4–8 weeks of treatment in all patients. The steady-state concentration correlated significantly with serum ribavirin clearance after multiple dosing (r = −0.875; 95% CI −0.932, −0.721; P < 0.001). Serum concentrations at weeks 4 and 8, Cmax and AUC(0,12 h) after multiple dosing and AUC(0,28 weeks) of ribavirin were significantly higher in sustained virological responders than in virological responders or nonresponders (P < 0.05, each). Increased Cmax and accumulation index of AUC(0,12 h) (median 10.5; 95% CI 6.4, 12.4), and prolonged washout half-life after multiple dosing reflected accumulation and slow clearance of ribavirin from the tissue compartments.
Conclusions
Continuous exposure and accumulation of ribavirin may be necessary for sustained virological response to combination therapy in chronic hepatitis C with genotype 1b and high viral load.
Keywords: chronic hepatitis C, genotype 1b, high viral load, interferon alpha 2b, pharmacokinetics, ribavirin
Introduction
Sustained virological response rates of less than 20% with interferon-alpha (IFN-α) monotherapy indicate the limit of this treatment in patients with chronic hepatitis C. One reason for this limit is hepatitis C virus (HCV)-related factors, which strongly and independently influence the outcome of IFN therapy. Specifically, patients infected with genotype 1 and high viral load exhibit markedly poor response to IFN therapy compared with those with other genotypes or low viral load [1, 2]. The addition of ribavirin, a synthetic nucleoside analogue with in vitro activity against several viruses [3], to IFN-α monotherapy has been reported to increase significantly the sustained virological response rates even in patients with IFN resistance arising from HCV-related factors, suggesting the potential synergistic antiviral effect of ribavirin [4, 5]. However, the antiviral efficacy of ribavirin when administered alone is unreliable against HCV. Ribavirin monotherapy can reduce serum liver-related enzyme concentrations, but not serum HCV RNA levels [6]. Thus, the exact antiviral mechanism by which ribavirin enhances IFN efficacy is not fully understood, although several mechanisms of action have been proposed, such as depletion of intracellular guanosine triphosphate pools, synthesis of viral messenger RNA with abnormal 5′ cap structures, inhibition of viral dependent RNA polymerase activity, and immunomodulatory effects on host immune responses [7–10].
Comparison of the early viral dynamics in patients treated with a combination of IFN-α with and without ribavirin showed that ribavirin appears to lack a direct synergistic antiviral effect, at least during the first 4 weeks of treatment [11]. However, a more recent study demonstrated a direct synergistic antiviral activity of ribavirin in the early viral dynamics under a high-dose IFN induction therapy [12]. From a pharmacokinetic point of view, there is no evidence of pharmacokinetic interactions between IFN-α and ribavirin [13, 14]. Furthermore, plasma ribavirin concentration requires at least several weeks after initial dosing to reach a steady state [13, 15], while the early viral dynamics occur within a few days of treatment [11, 12, 16]. Thus, the steady phase of ribavirin pharmacokinetics is beyond the first and second phase of viral dynamics, when viral dynamics has not been fully elucidated. These findings suggest that the synergistic antiviral activity of ribavirin may occur slowly at the later phase of treatment.
To reduce variation of virus-related factors and to address the most crucial subgroup of patients, we selected in the present study chronic hepatitis C patients with genotype 1b and high viral load, for whom cure with IFN monotherapy is difficult [1, 2]. We determined the pharmacokinetic profiles of ribavirin and IFN-α 2b in the combination therapy by single-dose and multiple-dose pharmacokinetic studies. We also investigated whether some pharmacokinetic parameters could be significantly associated with treatment outcome. Our results indicate that continuous exposure and accumulation of ribavirin may be necessary for sustained virological response to combination therapy in chronic hepatitis C with genotype 1b and high viral load.
Materials and methods
Patients
Twenty-seven patients with chronic hepatitis C of genotype 1b and high viral load were consecutively enrolled in this study conducted at Toranomon Hospital, Yamanashi Prefectural Central Hospital, and Musashino Red Cross Hospital, between April 2000 and May 2001. This study was a pharmacokinetics study of single and multiple doses of ribavirin and IFN-α 2b in combination therapy. The Ethics Review Committee of each institution approved the study protocol. Informed consent for participation was obtained from all patients before they entered the trial. Inclusion criteria were: (i) a positive test for anti-HCV antibody; (ii) HCV genotype 1b confirmed by polymerase chain reaction (PCR)-based method; (iii) serum HCV RNA levels ≥1.0 × 106 equivalent (Eq) ml−1 on a branched-DNA signal-amplification assay (Quantiplex HCV RNA version 2.0 Assay; Chiron, Emeryville, CA, USA; defined as ‘high’ viral load); (iv) persistently high concentrations of serum alanine transaminase (ALT) during the preceding 12 weeks; (v) confirmation of diagnosis of chronic hepatitis by liver biopsy specimens obtained within the preceding 48 weeks, as assessed by one pathologist, using the histological ranking system [17]; (vi) haemoglobin (Hb) concentration ≥12.0 g dl−1; (vii) platelet count ≥100 × 103 µl−1; (viii) bodyweight>60 kg; and (ix) age between 20 and 65 years. Exclusion criteria were: (i) liver cancer or severe liver failure, as described previously [2]; (ii) other forms of liver disease; (iii) coexisting serious medical or psychiatric illness; (iv) therapy with any other antiviral or immunomodulatory agent administered within the previous 24 weeks; (v) history of ribavirin therapy; (vi) patients with hepatitis B surface antigen or hepatitis B core antibody, as determined by radioimmunoassay; (vii) hypersensitivity to IFN or ribavirin; and (viii) pregnancy or lactation.
Study protocol and assessment
Treatment was provided for 24 weeks, with a subsequent 24-week follow-up period. The treatment schedule was as follows: on morning of day 1 (week 1), a single oral dose of 400 mg of ribavirin (two capsules each of 200 mg; Schering-Plough Int., Kenilworth, NJ, USA) and intramuscular IFN-α 2b (Schering-Plough) at a dose of 6 million units (MU) was administered simultaneously, followed by the first pharmacokinetic assessment. On day 1, in the evening and day 2, no ribavirin was administered, while IFN was administered every 24 h for the initial 2 weeks. Between day 3 (week 1) and day 6 (week 24), ribavirin was administered twice daily at a total daily dose of 800 mg. Following the 2-week induction therapy, IFN was administered three times a week for 22 weeks at a dose of 6 MU. On the morning of day 7 of week 24, the last dose of each agent was administered simultaneously, followed by the last pharmacokinetic assessment at week 28. During treatment, the dose of ribavirin was adjusted based on Hb concentration; ribavirin was reduced to 600 mg day−1 (as 200 mg in the morning and 400 mg in the evening) if Hb concentration fell below 10 g dl−1, and discontinued when it diminished below 8.5 g dl−1.
Biochemical and virological responses to treatment were assessed during the treatment period (weeks 1–24), and during the subsequent follow-up period (weeks 25–48). Biochemical response was defined as normalization of serum ALT activity by the end of treatment, and virological response was defined as undetectable serum HCV RNA by the end of treatment, using qualitative PCR assay (Amplicor HCV version 2.0; Roche Molecular Systems, Pleasanton, CA, USA) with a lower detection limit of 100 copies ml−1. Sustained biochemical or virological response was defined as normalization of serum ALT or as absence of serum HCV RNA, respectively, after completion of treatment until the end of the follow-up period (week 48).
The first pharmacokinetic studies were based on serum samples obtained sequentially during days 1–3. Specifically, 100- and 25-µl serum samples for measurement of serum ribavirin and IFN concentrations, respectively, were obtained before the initial single dose of both compounds (0 h) and then at 1, 3, 6, 12, 24, 36, and 48 h postdose. Subsequently, fasting blood samples were collected 1, 4, 8, 12, 16, and 20 weeks after the initial dosing. These fasting samples were obtained 12 h after preceding evening dosing and just before the morning dosing. The last pharmacokinetic studies were performed by using sequential serum samples obtained before the final multiple doses of both compounds (0 h) and then at 1, 3, 6, 12, 24, and 48 h postdose. Additional fasting serum samples were obtained on the morning once weekly during the first 4-week follow-up period (weeks 25–28). Serum ribavirin concentrations were determined by a validated high-performance liquid chromatography/tandem mass spectrometric assay using 13C-ribavirin as an internal standard [13, 18]. The assay was validated with respect to linearity within a range of 50.1–5005 ng ml−1, specificity, accuracy (within 15% for all runs), and precision (within 15% for all runs). The assay limit of quantification (LOQ) was 50 ng ml−1. Serum IFN-α 2b concentrations were measured by a validated sensitive electrochemiluminescence immunoassay with a LOQ of 1.3 IU ml−1 [14]. Individual serum ribavirin and IFN-α 2b concentration–time data were used for pharmacokinetic analysis by noncompartment model analysis [19]. Maximum concentration (Cmax) and time to maximum concentration (Tmax) were the observed values. Terminal phase half-life was calculated using the method of least-squares regression of the data obtained at 12–48 h and 3–24 h in the initial single dose of ribavirin and IFN-α 2b, respectively, and at 25–28 weeks and 3–24 h in the final multiple dose of both, respectively. The areas under the serum concentration–time curve from time 0 to 12 h [AUC(0,12 h)] and from week 0 to 28 (AUC[0,28 weeks]) for ribavirin, and from time 0 to 24 h[AUC(0,24 h)] for IFN-α 2b was calculated using the linear trapezoidal method. The apparent serum ribavirin clearances (CL/F) at single and multiple doses were calculated as the daily single dose divided by AUC(0,∞) and AUC(0,12 h), respectively. Correlation between CL/F and ribavirin concentration was examined using Pearson and least-squares regression analyses. The steady-state phase of ribavirin was determined by visual inspection of the concentration–time profile curves. The steady-state concentration was calculated as mean of concentrations during the steady-state phase. Accumulation indices (R) for IFN-α 2b and ribavirin were calculated as AUC(0,24 h) or AUC(0,12 h) (final multiple dose) divided by AUC(0,24 h) or AUC(0,12 h) (initial single dose), respectively.
Clinical and laboratory data were assessed twice weekly during the first 2 weeks, at least once weekly during the next 2 weeks, and at least every 4 weeks during the remaining treatment period and 24-week follow-up period. Adverse effects were monitored clinically by careful interview and medical examination throughout the study. Patient compliance with treatment was evaluated by a questionnaire and medical records and by counting the number of returned capsules.
Statistical analysis
Tukey's multiple analysis was used to assess the differences in pharmacokinetic parameters between treatment efficacies. Treatment outcomes were analysed on an intention-to-treat basis. Correlation between ribavirin concentrations and sustained virological response rates was analysed by H-test. The criterion for statistical significance was a P-value of <0.05. All calculations were performed using the SAS program version 6.12 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics at baseline are summarized in Table 1. Twenty-seven patients (24 males and three females) between the ages of 28 and 60 years (median 49) and weighing between 60.3 and 87.0 kg (67.6) participated in the study. All but one patient fully completed the treatment and were followed up as scheduled. Patient compliance with ribavirin was excellent. All patients experienced adverse effects during treatment, and showed profiles similar to previously reported symptoms [4, 5]. Only one patient developed severe treatment-related adverse events (myalgia and anorexia) that led to discontinuation of therapy. Treatment reduced Hb concentrations in all patients from a median value of 14.9 (range 12.1–17.5) to 10.9 (8.8–13.6) g dl−1, representing a fall of 1.1–6.7 g dl−1 (median 4.0). The dose of ribavirin was reduced to 600 mg day−1 in 10 of the 27 patients because of falls in Hb concentrations to less than 10.0 g dl−1 between weeks 3.4 and 17.2 (median 12.1).
Table 1.
Baseline profiles of the patients.
| Demography | |
| Gender (F/M) | 3/24 |
| Age (years) | 28–60 (49)*1 |
| Body weight (kg) | 60.3–87.0 (67.6)*1 |
| Ribavirin dose/body weight ratio (mg kg−1) | 9.2–13.3 (11.8)*1 |
| Laboratory data | |
| ALT (U L−1) | 47–199 (84)*1 |
| Creatinine (mg dL−1) | 0.7–1.4 (1.0)*1 |
| Ferritin (ng mL−1) | 23–560 (110)*1 |
| Hemoglobin (g dL−1) | 12.1–17.5 (14.9)*1 |
| Liver histology | |
| Stage (1/2/3/4) | 14/12/1/0 |
| Grade (mild/moderate/severe) | 10/17/0 |
| Virology | |
| Viral load (Eq mL−1 × 106) | 1.3–25 (11)*1 |
ALT, alanine transaminase; Eq, equivalents.
Numbers in parentheses denote median. Normal reference ranges: 6–40 U L−1 for ALT; 0.6–1.1 for creatinine; 10–190 for ferritin; 13.0–17.0 for hemoglobin (male), 11.3–15.0 for hemoglobin (female).
Biochemical and virological responses to therapy
During treatment, 22 (81%) patients showed a biochemical response. After cessation of treatment, 16 (59%) patients achieved a sustained biochemical response. Serum ALT activities in six relapsers returned to the baseline level. By the end of treatment, 21 (78%) patients exhibited virological response. After cessation of treatment, five (19%) patients attained sustained virological response (SVR). Serum HCV RNA levels in 16 virological responders returned to baseline values after cessation of treatment. Of ten patients in whom ribavirin was reduced, seven were virological responders and one achieved SVR.
Pharmacokinetics of ribavirin
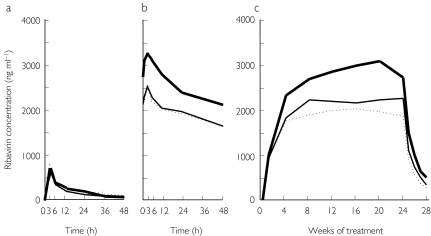
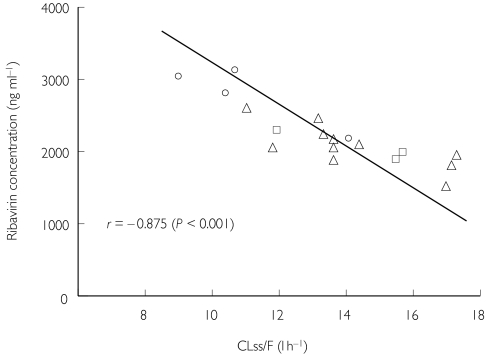
Pharmacokinetic parameters in the initial single dose and the final multiple dose of ribavirin are summarized in Table 2. Figure 1 shows ribavirin concentration–time profiles with the single and multiple doses. Following the initial single-dose administration, peak serum concentrations (Cmax) occurred between 1 and 6 h, and rapidly decreased to 56–193 ng ml−1 at 48 h. There was no significant difference in Cmax or AUC(0,12 h) between patients. The multiple-dose pharmacokinetics (day 7, week 24) represented rapid absorption and distribution phases again in a similar manner (Figure 1). Cmax and AUC(0,12 h) values of SVR patients were significantly higher than those of virological responders or nonresponders (P < 0.05 for both). Half-lives following multiple dosing were prolonged compared with those following single dosing. There were measurable concentrations between 127 and 729 ng ml−1 in all patients even at the end of week 28. Bioavailability increased markedly, with the ratio of week 24 to week 1 AUC(0,12 h) (RAUC). SVR patients were higher in RAUC than others, though not statistically significantly so. There was no significant difference in the above pharmacokinetics between patients with and without reduction of ribavirin. CL/F in the initial single dose was 23.9–47.2 l h−1 (median 35.7; 95% confidence interval [CI] 25.2, 44.8), and was not significantly associated with treatment outcome. Following multiple dosing, CL/F (CL steady-state [ss]/F) decreased to 9.0–17.3 (13.7; 10.1, 15.4). SVR patients were significantly lower in CLss/F than nonresponders (P < 0.05). The CLss/F values were closely correlated with ribavirin concentrations at steady-state phase (r = −0.875; 95% CI −0.932, −0.721; P < 0.001, Figure 2).
Table 2.
Ribavirin pharmacokinetic parameters in the single and multiple dose phases.
| Initial single dose | Final multiple dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tmax(hr) | Cmax (ng mL−1) | AUC(0,12 hr)(μg.hr mL−1) | T1/2 (hr) | Tmax (hr) | Cmax*†(ng mL−1) | AUC(0,12 hr)*†(μg.hr mL−1) | T1/2 (hr) | RAUC | |
| Sustained virological responders (n =5) | 3.0 (3.0–3.0) | 660 (399–1013) | 4.1 (2.8–6.6) | 30.6 (23.8–41.4) | 3.0 (1.1–6.5) | 3484 (2645–4209) | 37.8 (29.3–43.9) | 305 (243–387) | 10.5 (6.4–12.4) |
| Virological responders (n =16) | 3.0 (2.2–3.4) | 576 (528–674) | 3.7 (3.5–4.5) | 26.8 (23.3–30.7) | 3.0 (1.8–7.6) | 2712 (2413–2795) | 28.7 (25.5–29.3) | 284 (261–315) | 7.1 (6.8–8.2) |
| Non-responders (n =6) | 3.0 (3.0–3.0) | 814 (483–1097) | 4.5 (3.1–6.3) | 28.7 (15.8–49.2) | 3.0 (3.0–3.0) | 2696 (1767–3295) | 26.1 (18.3–34.9) | 320 (260–346) | 9.2 (7.2–10.0) |
| Total (n =27) | 2.0 (2.5–3.3) | 628 (579–741) | 4.1 (3.8–4.8) | 27.3 (25.3–32.9) | 3.0 (2.5–5.9) | 2734 (2508–2982) | 28.9 (26.5–31.5) | 299 (277–317) | 8.3 (7.4–8.8) |
Values expressed as median. (95% confidence interval). Accumulation index (R) = AUC of week 24 / AUC of week 1.
P <0.05 for sustained virologic responders vs. virologic responders.
P <0.05 for sustained virologic responders vs. non-responders.
Figure 1.
Mean ribavirin concentration–time profiles in the initial single-dose (a) and final multiple-dose (b) studies and steady-state phase (c) in patients treated with interferon-alpha 2b plus ribavirin combination therapy. —, Patients with sustained virological response; —, those with virological response during treatment, but without sustained virological response after cessation of treatment; ·····, patients without virological response throughout the study period.
Figure 2.
Correlation between apparent serum clearance of ribavirin following multiple dosing (CLss/F) and serum ribavirin concentration at steady-state phase. ○, Patients with sustained virological response; Δ, those with virological response during treatment, but without sustained virological response after cessation of treatment; patients without virological response throughout the study period.
Following multiple dosing, nearly trough concentrations began to asymptote generally by 4–8 weeks of ribavirin administration (Table 3 and Figure 1). The asymptotic levels varied relatively widely from patient to patient. The steady-state assessment between weeks 4 or 8 and 24 indicated that SVR patients constantly maintained higher values than other patients at each time point. Specifically, the concentrations at weeks 4 and 8 were significantly higher in SVR patients than in others (P < 0.05, each). There was no significant difference in ribavirin concentration between patients with and without reduction of ribavirin. SVR rate at week 4 was 100% (2/2), 33% (2/6), 6% (1/16), and 0% (0/3) at ribavirin concentrations of 2500 to <3000, 2000 to < 2500, 1500 to < 2000, and < 1500 ng ml−1, respectively (P < 0.05). At the concentration of ≥ 2000 ng ml−1, SVR reached 50% (4/8), compared with 5% (1/17) at < 2000 ng ml−1 (P < 0.05). SVR rate at week 8 was 100% (2/2), 14% (1/7), and 20% (2/10) at ≥ 3000, 2500 to < 3000, and 2000 to < 2500 ng ml−1, respectively. None attained SVR at less than 2000 ng ml−1 (P < 0.05). AUC (0–28 weeks) values of 52 507–75 871 µg.week ml−1 (median, 67 227; 95% CI 54 106, 78 710) in SVR patients tended to be higher than those of 36 543–62 580 µg.week ml−1 (49 677; 46 252, 53 916) in virological responders, though not statistically significantly so, and those of 30 486–62 967 µg.week ml−1 (48 355; 36 014, 59 910) in nonresponders (P < 0.05).
Table 3.
Ribavirin concentration-time profiles in patients treated with interferon alpha-2b plus ribavirin combination therapy.
| Serum ribavirin concentrations (ng mL−1) | |||||||
|---|---|---|---|---|---|---|---|
| 1 wk | 4 wk*† | 8 wk*† | 12 wk | 16 wk | 20 wk | 24 wk | |
| Sustained virological responders (n = 5) | 1025 (719–1281) | 2484 (1799–2799) | 2509 (2269–3093) | 2765 (2273–3401) | 2913 (2431–3517) | 3475 (2188–3966) | 2751 (2034–3354) |
| Virological responders (n = 16) | 906 (823–1049) | 1789 (1717–2079) | 2077 (2055–2417) | 2117 (1937–2317) | 2069 (1810–2392) | 2295 (1967–2367) | 2158 (1970–2318) |
| Non-responders (n = 6) | 856 (734–1038) | 1575 (1376–1792) | 1989 (1401–2557) | 1925 (1483–2639) | 2093 (1555–2703) | 2053 (1397–2657) | 2101 (1507–2711) |
| Total (n = 27) | 903 (858–1016) | 1744 (1747–2059) | 2231 (2057–2439) | 2149 (2053–2453) | 2224 (2071–2481) | 2289 (2068–2564) | 2169 (2055–2427) |
Values expressed as median (95% confidence interval).
P < 0.05 for sustained virological responders vs. virological responders.
P < 0.05 for sustained virological responders vs. non-responders.
Pharmacokinetics of IFN
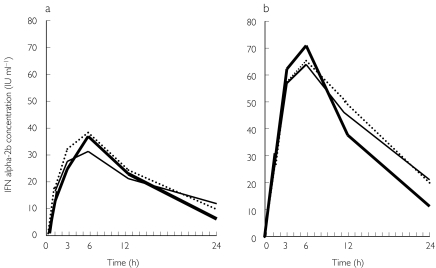
The initial single dose and final multiple dose pharmacokinetics of IFN-α 2b also showed rapid absorption and distribution phases within 24 h (Figure 3). Cmax occurred between 3 h and 12 h with both single dose and multiple doses (Table 4). Accumulation rate (RAUC) values based on the ratio of week 1 and week 24 AUC(0,24 h) values ranged from 1.0 to 6.0. Following multiple dosing, serum concentrations did not asymptote and fluctuated widely below the Cmax value observed after the final multiple dose. Serum concentrations at 24 h after the final dosing did not significantly differ from those after the single dose. The concentration at weeks 24–28 during follow-up was not measurable in most patients. The values were characterized by very high variability between individual patients or between time points. There was no significant correlation between the IFN pharmacokinetic parameters and response to the combination therapy.
Figure 3.
Interferon-alpha (IFN-α) 2b concentration–time profiles in the initial single-dose (a) and final multiple-dose (b) studies in patients treated with IFN-α 2b plus ribavirin combination therapy. —, Patients with sustained virological response; —, those with virological response during treatment, but without sustained virological response after cessation of treatment; ·····, patients without virological response throughout the study period.
Table 4.
Interferon alpha-2b pharmacokinetic parameters in the single and multiple dose phases.
| Initial single dose | Final multiple dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Tmax (hr) | Cmax (IU mL−1) | AUC(0,24 hr) (IU.hr mL−1) | T1/2 (hr) | Tmax (hr) | Cmax (IU mL−1) | AUC(0,24 hr) (IU.hr mL−1) | T1/2 (hr) | RAUC | |
| Sustained virological responders (n = 5) | 6.0 (6.0–6.0) | 37.8 (30.1–43.7) | 480 (431–535) | 5.1 (3.0–10.6) | 6.0 (3.8–7.0) | 60.6 (32.6–109.8) | 900 (592–1346) | 6.2 (5.5–24.4) | 1.8 (1.6–2.7) |
| Virological responders (n = 16) | 6.0 (4.4–6.8) | 29.1 (25.7–42.5) | 401 (357–569) | 8.4 (7.0–9.4) | 6.0 (4.8–6.0) | 65.7 (51.7–80.7) | 896 (714–1338) | 9.3 (6.8–17.4) | 2.3 (2.0–2.6) |
| Non-responders (n = 6) | 6.0 (4.2–6.8) | 32.5 (25.8–57.0) | 482 (379–797) | 8.6 (6.5–10.5) | 6.0 (3.5–9.5) | 56.6 (36.4–101.4) | 869 (594–1694) | 12.0 (7.9–19.1) | 1.7 (1.3–4.5) |
| Total (n = 27) | 6.0 (5.0–6.4) | 32.0 (30.0–41.4) | 454 (411–559) | 8.3 (7.1–8.9) | 6.0 (5.0–6.4) | 61.2 (56.3–78.5) | 883 (794–1226) | 9.3 (8.6–15.8) | 2.0 (1.9–2.7) |
Values expressed as median. (95% confidence interval). Accumulation index (R) = AUC of week 24 / AUC of week 1.
Discussion
There are only a few studies on 24-week dosing pharmacokinetics of ribavirin in Asian patients. The concentration–time profile of ribavirin after initial dosing was closely similar to that described previously [13, 14, 20, 21]. Moreover, a similar curve was also observed after 24 weeks, with rapid absorption and distribution phases and a long-terminal clearance phase. These findings indicate that ribavirin pharmacokinetics is not essentially affected by race or IFN treatment regimen. The lack of differences in the single-dose pharmacokinetics among our patients indicates that the single-dose pharmacokinetic profile does not influence or predict the multiple-dose pharmacokinetics or response to the combination therapy. Cmax, AUC(0,12 h), and the longer washout half-life after multiple dosing, AUC(0,28 week), and RAUC imply that ribavirin accumulates and is cleared slowly from the tissue compartments [22, 23]. These parameters were higher in SVR patients, indicating that sustained viral eradication might require some degree of exposure and accumulation of ribavirin with its slowly acting antiviral or immune-mediated activity [9, 10].
Our study reconfirmed that serum ribavirin concentrations take at least 4 weeks to reach a steady state, and that the steady-state concentrations are maintained consistently from week 4–8 to the end of treatment [13, 15], irrespective of the response to the combination therapy. Such a steady-state phase could be interpreted to mean that an equilibrium is reached between intra- and extra-cellular concentrations. In cells attaining saturation point, phosphorylated ribavirin could deplete intracellular guanosine triphosphate pools, synthesize viral messenger RNA with abnormal 5′ cap structures, and inhibit viral-dependent RNA polymerase activity [7, 8]. In the extracellular space, ribavirin could develop HCV-specific T-cell responses and modulate the balance of Th1–Th2 cytokine responses [9, 10]. These actions of ribavirin may depend on intra- and extra-cellular ribavirin concentrations. In terms of viral kinetics, the time when the concentrations reach steady state (week 4–8) is beyond the first, rapid phase and the second, slower phase of viral decline, which largely occur within several days of treatment [11, 12, 16]. Thus, ribavirin may not contribute directly to antiviral activity in the early viral dynamic phase. Although it is unknown whether a further slower phase of viral decline may occur beyond the initial two phases, ribavirin may alter viral replication kinetics in the later phase, thereby precipitating clearance of HCV-infected cells. This suggestion supports the recent study that ribavirin lacks direct synergistic antiviral effects, at least in the early period of combination therapy [11].
Of note, a critical and stable level of steady-state concentration of serum ribavirin seems to be required to achieve SVR in patients with genotype 1b and high viral load. Certainly, the higher the serum ribavirin concentrations became, the higher the SVR rates became. However, it remains to be determined when ribavirin concentrations should be measured, or how high levels should be maintained to predict or produce SVR. In a recent study [18], plasma concentrations at week 4 were shown to be one of the major factors associated with favourable outcome, although concentrations at other time points were not analysed and not compared with those at week 4. Our results demonstrate that the concentrations at weeks 4 and 8 seemed to be significantly associated with SVR by coincidentally comparing those at other time points. The mechanism through which variation in the ribavirin concentration between patients arose was not clear because no factor could be identified affecting the week 4 concentration. Our results show that one possible factor was apparent serum ribavirin clearance at the steady-state phase (CLss/F) in affecting the steady-state concentration. Previous studies showed that the CL/F is around 20 l h−1 with a 4-week treatment interval [14, 18]. Thus, our CLss/F values seem to be a decreasing and time-dependent pharmacokinetic factor, although there is no definitive explanation for the phenomenon. The determinant of CLss/F remains to be established, although several clinical factors have been shown partly to influence the CLss/F [18]. It should be cautioned, however, that although the use of higher ribavirin concentrations may increase the rate of SVR, they may also aggravate haemolytic anaemia.
A recent study reported that the week 4 concentration was significantly related to treatment outcome even in patients with genotype non-1 and those who received 48-week combination therapy [18]. However, it is possible that ribavirin concentrations might be less associated with treatment outcome in genotype 2 patients in Japan, where the IFN treatment regimen in use is different from that in the West. The combined treatment with high-dosage or induction therapy of IFN could lessen the utility of ribavirin in genotype 2 patients, who are sensitive to IFN monotherapy. Because the above findings did not hold true for all patients, SVR patients with low ribavirin concentrations may possibly represent the effects of IFN therapy per se. Conversely, some patients showing relatively higher concentrations did not attain SVR. Some appropriate conditions, including unknown host- and HCV-related factors, also seem to be important for achieving SVR.
Pharmacokinetics for IFN-α 2b showed no or little change in absorption, distribution and clearance rates, although AUC values increased approximately two-fold following multiple dosing. Our pharmacokinetic results coincided with those reported previously in a 4-week dosing study [13]. This suggests that the IFN treatment regimen does not alter IFN pharmacokinetic profile and that treatment duration and dosage of ribavirin also do not affect IFN pharmacokinetics. The above findings could indirectly support the fact that the mechanism of enhanced antiviral activity of combination therapy does not involve alteration of the pharmacokinetics of one or both compounds [13, 14]. Serum IFN concentrations were relatively lower and fluctuated throughout treatment and could not be measured at prefinal dosing. Taken together, increased IFN RAUC values do not signify accumulation of the compound, partly reflecting a decline in the efficiency of uptake and spillover of IFN due to down-regulation of cellular IFN receptors [13, 14]. No IFN pharmacokinetic parameter analysed in our study was found to affect decisively the response to combination therapy, while the IFN treatment regimen had a major impact on the treatment outcome [24].
In conclusion, our results of single-dose and multiple-dose pharmacokinetics of IFN-α 2b and ribavirin in combination therapy indicate that accumulation and exposure to ribavirin at some critical and stable level may be required to achieve SVR in patients infected with genotype 1b and high viral load. A further study is required to identify the optimal steady-state concentration of ribavirin to achieve SVR and to develop a more effective and rational treatment based on serum ribavirin concentrations.
Acknowledgments
We are indebted to Schering-Plough (Osaka, Japan) for their kind help. We are also grateful to Mr Misao Iba (Assistant Product Manager, Schering-Plough), Mr Takao Harada (Department Manager, Advanced Scientific Information, Schering-Plough), and Mr Takayuki Yoshioka (Drug Metabolism and Pharmacokinetics Research & Development Division) for assistance with statistical analysis. Supported in part by research contracts from Schering-Plough International.
References
- 1.Lau JYN, Davis GL, Kniffen J, et al. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993;341:1501–1504. doi: 10.1016/0140-6736(93)90635-t. [DOI] [PubMed] [Google Scholar]
- 2.Tsubota A, Chayama K, Ikeda K, et al. Factors predictive of response to interferon-alfa therapy in hepatitis C virus infection. Hepatology. 1994;19:1088–1094. [PubMed] [Google Scholar]
- 3.Patterson JL, Fernandez-Larsson R. Molecular mechanisms of action of ribavirin. Rev Infect Dis. 1990;12:1139–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 4.McHutchison JG, Gordon SC, Schiff ER, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med. 1998;339:1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 5.Davis GL, Esteban-Mur R, Rustgi V, et al. Interferon alfa-2b alone or in combination with ribavirin for the treatment of relapse of chronic hepatitis C. N Engl J Med. 1998;339:1493–1499. doi: 10.1056/NEJM199811193392102. [DOI] [PubMed] [Google Scholar]
- 6.Di Bisceglie AM, Conjeevaram HS, Fried MW, et al. Ribavirin as therapy for chronic hepatitis C. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1995;123:897–903. doi: 10.7326/0003-4819-123-12-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 7.Sidwell R, Huffman JH, Khare L, Allen LB, Witlowski JT, Robins RK. Broad-spectrum activity of virazole 1-beta-d-ribofuranosyl-1,2,-triazole-3-carboxamide. Science. 1972;117:705–706. doi: 10.1126/science.177.4050.705. [DOI] [PubMed] [Google Scholar]
- 8.Patterson JL, Fernandez-Larson R. Molecular mechanisms of action of ribavirin. Rev Infect Dis. 1990;12:1132–1146. doi: 10.1093/clinids/12.6.1139. [DOI] [PubMed] [Google Scholar]
- 9.Ning Q, Brown D, Parodo J, et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1 and the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol. 1998;160:3487–3493. [PubMed] [Google Scholar]
- 10.Cramp ME, Rossol S, Chokshi S, Carucci P, Williams R, Naoumov NV. Hepatitis C virus-specific T-cell reactivity during interferon and ribavirin treatment in chronic hepatitis C. Gastroenterology. 2000;118:346–355. doi: 10.1016/s0016-5085(00)70217-4. [DOI] [PubMed] [Google Scholar]
- 11.Zeuzem S, Schmidt JM, Lee JH, von Wagner M, Teuber G, Roth WK. Hepatitis C virus dynamics in vivo: effect of ribavirin and interferon alfa on viral turnover. Hepatology. 1998;28:245–252. doi: 10.1002/hep.510280132. [DOI] [PubMed] [Google Scholar]
- 12.Asahina H, Izumi N, Uchihara M, et al. A potent antiviral effect on hepatitis C viral dynamics in serum and peripheral blood mononuclear cells during combination therapy with high-dose daily interferon alfa plus ribavirin and intravenous twice-daily treatment with interferon beta. Hepatology. 2001;34:377–384. doi: 10.1053/jhep.2001.26086. [DOI] [PubMed] [Google Scholar]
- 13.Khakoo S, Glue P, Grellier L, et al. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998;46:563–570. doi: 10.1046/j.1365-2125.1998.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glue P, Rouzier-Panis R, Raffanel C, et al. A dose-ranging study of regulated interferon alfa-2b and ribavirin in chronic hepatitis C. Hepatology. 2000;32:647–653. doi: 10.1053/jhep.2000.16661. [DOI] [PubMed] [Google Scholar]
- 15.Lertora JJ, Rege AB, Lacour JT, et al. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1991;50:442–449. doi: 10.1038/clpt.1991.162. [DOI] [PubMed] [Google Scholar]
- 16.Nuemann AU, Lam NP, Dahari H, et al. Hepatitis C viral dynamics in vivo and the antiviral efficacy of interferon-α therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 17.Tsubota A, Kumada H, Chayama K, et al. Time course of histological changes in patients with a sustained biochemical and virological response to interferon-alfa therapy for chronic hepatitis C virus infection. J Hepatol. 1997;27:49–55. doi: 10.1016/s0168-8278(97)80279-6. [DOI] [PubMed] [Google Scholar]
- 18.Jen JF, Glue P, Gupta S, Zambas D, Hajian G. Population pharmacokinetic and pharmacodynamic analysis of ribavirin in patients with chronic hepatitis C. Ther Drug Monit. 2000;22:555–565. doi: 10.1097/00007691-200010000-00010. [DOI] [PubMed] [Google Scholar]
- 19.Gibaldi M, Perrier D. Pharmacokinetics. 2. New York: Marcel Dekker; 1982. pp. 409–449. [Google Scholar]
- 20.Laskin OL, Longstreth JA, Hart CC, et al. Ribavirin disposition in high risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987;41:546–555. doi: 10.1038/clpt.1987.70. [DOI] [PubMed] [Google Scholar]
- 21.Paroni R, Del Puppo M, Borghi C, Sirtori CR, Galli Kienle M. Pharmacokinetics of ribavirin and urinary excretion of the major metabolite 1,2,4-triazole-3-carboxamide in normal volunteers. Int J Clin Pharmacol Ther Toxicol. 1989;27:302–307. [PubMed] [Google Scholar]
- 22.Catlin DH, Smith RA, Samuels AI. 14C-Ribavirin: distribution and pharmacokinetic studies in rats, baboons and man. In: Smith RA, Kirkpatrick W, editors. Ribavirin. A Broad Spectrum Antiviral Agent. New York: Academic Press; 1980. pp. 83–98. [Google Scholar]
- 23.Ferrara EA, Oishi JS, Wannemacher RW, Jr, Stephen EL. Plasma disappearance, urine excretion and tissue distribution of ribavirin in rats and rhesus monkeys. Antimicrob Agents Chemother. 1981;19:1042–1049. doi: 10.1128/aac.19.6.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsubota A, Chayama K, Arase Y, et al. Factors useful in predicting the response to interferon therapy in chronic hepatitis C. J Gastroenterol Hepatol. 1993;8:535–539. doi: 10.1111/j.1440-1746.1993.tb01648.x. [DOI] [PubMed] [Google Scholar]