The antiepileptic drug hypersensitivity syndrome (AHS) is associated with treatment with aromatic antiepileptic drugs. We describe a case in which AHS followed exposure to carbamazepine (CBZ) and, unusually, recurred during treatment with valproate.
A 36-year-old man was admitted because of fever, rash and lymphadenopathy. He had been well until 32 days earlier when epilepsy was diagnosed and CBZ treatment started. Two weeks before admission he complained of toothache. Examination revealed left cervical lymphadenopathy and clarithromycin was prescribeded. Five days before admission he consulted because of rash. Fever and left submaxillary lymph nodes were noted. Clarithromycin was discontinued. Worsening of the rash (Figure 1) led to his admission. He denied other symptoms. There was no history of exposure to tuberculosis or of drugs allergies. His temperature was 37.8°C. There was an erythematous maculo-papular rash on his arms, thighs and trunk and oronasal ulcers with features of Stevens–Johnson syndrome. Conjunctival erythema, pharyngitis and bilateral cervical and submaxillary lymphadenopathy were present. Full blood count revealed lymphocytosis (8000 mm−3) with slight eosinophilia (500 mm−3). A computed tomographic scan revealed a 1.5 cm right parahilar node. Biopsy of a cervical node showed follicular hyperplasia. Other investigations including blood cultures, HIV, cytomegalovirus, Epstein-Barr virus, hepatitis B and C virus, Brucella sp., Rickettsia sp. (IgG), Borrelia sp. and Leishmania sp. and tuberculin test were negative.
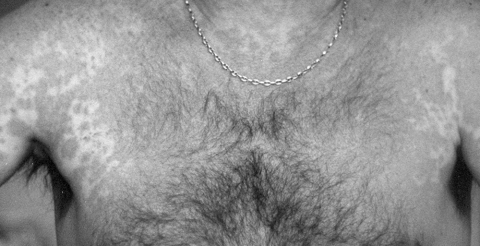
Figure 1.
Exanthematous eruption; red, symmetrical and widespread.
Treatment with CBZ was discontinued and methylprednisolone started. The patient improved. Two weeks later, lymphadenopathy had resolved and valproate was started. Immediately a widespread exanthematous rash similar his previous one, fever and adenopathy recurred. Skin biopsy revealed findings consistent with Stevens–Johnson syndrome. Valproate was discontinued. Patch testing to CBZ and valproic acid performed 4 months later was positive for each . He is now being treated with gabapentin and remains well.
The incidence of AHS is 1 per 1-10 thousand patients treated with aromatic anticonvulsants. Onset usually occurs within 3 months of initiating treatment [1], but patients who are rechallenged can develop the symptoms almost immediately. The rash is commonly an exanthem, but more severe reactions including Stevens–Johnson syndrome and toxic epidermal necrolysis also occur [2].
Phenytoin, CBZ and phenobarbitone each contain an aromatic ring that is metabolized to reactive arene oxides by cytochrome P450. Further metabolism involves epoxide hydrolase. It has been postulated that deficient reduction by epoxide hydrolase leads to accumulation of reactive intermediates [3]. These may bind tissue macromolecules causing cell damage or act as haptens stimulating T-cells and triggering a systemic autoimmune response [4].
Valproic acid is not an aromatic compound. To our knowledge, only two cases of AHS caused by valproate have been reported previously [5, 6]. The mechanism is unknown. The present case, in which symptoms recurred rapidly after valproate was initiated in a patient who had recently recovered from CBZ-associated AHS, is consistent with an immunological mechanism, such as a reaction to circulating antibodies to antiepileptic drugs probably triggered by a clinical cross-reactivity between CBZ and valproate.
References
- 1.Licata AL, Louis ED. Anticonvulsant hypersensitivity syndrome. Comprehens Ther. 1996;22:152–155. [PubMed] [Google Scholar]
- 2.Vittorio C, Muglia J. Anticonvulsant hypersensitivity syndrome. Arch Intern Med. 1995;155:2285–2290. [PubMed] [Google Scholar]
- 3.Smythe MA, Umstead GS. Phenytoin hepatotoxicity: a review of the literature. Drug Intell Clin Pharm. 1989;29:13. doi: 10.1177/106002808902300102. [DOI] [PubMed] [Google Scholar]
- 4.Potter T, Di Gregorio F, Stiff M, Hashimoto K. Dilantin hypersensitivity syndrome initiating staphylococcus toxic shock. Arch Dermatol. 1994;130:856–858. [PubMed] [Google Scholar]
- 5.Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235–1238. doi: 10.1046/j.1365-2133.2001.04240.x. [DOI] [PubMed] [Google Scholar]
- 6.Platin P, Cartier H, Le Bihan G, Clovard P, Lellovche F, Leroy JP. Syndrome d’hipersensibilité médicamenteuse au cours d’un traitment par acide valproïque. Presse Med. 1995;24:1624. [PubMed] [Google Scholar]