Abstract
Aims
To compare variability of heart rate-corrected QT intervals (QTc) using three different methods in a study of low-dose oral haloperidol.
Methods
In a randomized, double-blind, placebo-controlled, crossover trial, we studied QT interval pharmacodynamics of single doses of oral haloperidol (10 mg) in 16 healthy subjects. Heart rate correction of the QT interval was performed using Bazett’s, Fridericia's and subject-specific correction methods. The subject-specific correction was performed using linear mixed modelling of placebo period QT vs RR data from each study subject.
Results
The subject-specific correction, in the form of QTc = QT/RRα, yielded a correction term α (slope of the log-transformed QT vs RR relationship) that ranged from 0.23 to 0.38 in individual subjects, i.e. the fixed correction term α = 0.5 of Bazett's correction was outside and the fixed correction term α = 0.33 of Fridericia's correction inside the range of individual values. The mean absolute slope of the QTcvs RR regression line using the subject-specific correction was significantly lower than the mean slopes obtained using either Bazett's or Fridericia's corrections. All three methods revealed a statistically significant greater mean QTc on haloperidol than on placebo at 10 h post-drug administration. The mean QT (95% CI) was 421.6 (410.8, 432.4), and 408.4 (398.6, 417.8) on haliperidol and placebo, respectively, using the subject-specific correction method (P = 0.0053). The mean QTc (95% CI) was 425.4 (414.3, 436.5) and 403.1 (394.3, 411.9) on haliperidol and placebo, respectively, using Bazett's correction (P = 1.7 × 10−5) and 423.1 (412.6, 433.6) and 408.2 (398.6, 417.8) on haliperidol and placebo, respectively, using Fridericia's correction (P = 7.7 × 10−4). Raw P-values were calculated using a paired t-test. Bonferroni-corrected P-values were calculated by multiplying the raw P-values by 13.
Conclusion
Haloperidol caused a statistically significant mean QTc prolongation using the three correction methods. The QTc intervals were less dependent on RR intervals using the subject-specific method, thus decreasing the possibility of over- or under-correction. The interindividual QTc changes from baseline varied significantly depending on the method of correction used.
Keywords: haloperidol, heart rate correction, QT interval
Introduction
Prolongation of the heart rate-corrected QT interval (QTc) as a biomarker for malignant drug-induced ventricular torsades de pointes tachycardia has drawn attention from many drug developers and regulatory agencies. The uncorrected QT interval shortens with increases in heart rate. Therefore, when testing the QT-prolonging potential of a drug known to alter heart rate, it is difficult to ascertain whether an observed QT change is due to a drug repolarization effect or a function of a change in heart rate. Heart rate correction of the QT interval is needed in such situations and many formulae have been proposed since the early ones proposed by Bazett and Fridericia [1, 2]. The optimal heart rate correction of the QT interval is arguable and has been a topic of discussion for many years.
QT intervals measured during drug treatment and in its absence can vary widely depending on the heart rate correction method used, leading to contrasting conclusions on the QT-prolonging potential of an investigated drug. For instance, β-blocker-induced mean QTc change has been shown to vary from −12 ms to +17 ms, while amiodarone-induced mean QTc change has been shown to vary from +13 ms to +31 ms dependent on the method used for heart rate correction [3]. Similarly, QTc intervals can vary up to 34 ms in children at rest dependent on the correction method used [4]. While this magnitude of QTc change may not be meaningful in a single patient and often rightly disregarded by practising clinicians, it draws substantial attention from both the pharmaceutical industry and regulatory agencies when averaged in a large group of individuals. A mean QTc change of as little as 10 ms may indicate a ‘signal’ that a drug may carry an arrhythmic liability. Sertindole was removed from the market in Europe because of a mean drug-induced QTc prolongation of 22 ms at therapeutic doses and reports of unexplained deaths as well as nonfatal arrhythmias [5]. Even more minute QTc prolongation in the region of 5 ms was reported with therapeutic doses of terfenadine before its removal from the US market [6].
The Bazett correction is commonly used in clinical settings because of its ease of use, and involves dividing the uncorrected QT (in seconds) by the square root of the RR interval (in seconds). The limitations of this method include over-correction at faster heart rates (lower RR intervals) and under-correction at slower heart rates (higher RR intervals), and these are well described in the literature [7, 8].
Besides the problem of over- and under-correction that may accompany use of uniform heart rate correction methods such as Bazett's or Fridericia’s, the QT vs RR relationship is highly variable interindividually and therefore it does not seem intuitive to apply a standardized correction formula to such a varying relationship. Some of the major factors influencing the QT vs RR relationship include sex, age, diurnal variation and autonomic activity [9–11]. Using ebastine, a nonsedating antihistamine, as an example, Malik has demonstrated the potential for making type I statistical errors with standardized heart rate correction methods such as Bazett's formula [12]. Ebastine is used therapeutically at doses of 10–20 mg but causes heart rate acceleration when used in high doses. Using Bazett's formula, ebastine was reported to cause nonsignificant QTc prolongation at a 60-mg dose, and statistically significant QTc prolongation at a 100-mg dose. However, using individualized regression analysis, QTc was not prolonged at all at either of these doses. Terfenadine was used as a positive control and showed prolongation in QTc with both heart rate correction methods.
In the present study, we employed individual regression analysis to correct the QT interval for heart rate and evaluated the QT-prolonging potential of haloperidol. Haloperidol is a commonly used antipsychotic drug that has potential to block IKr channels in a concentration-dependent manner in vitro[13]. While there are a number of case reports in the literature that describe haloperidol-induced QT prolongation [14, 15] and torsades de pointes arrhythmias [16], the ability of haloperidol to prolong the QT interval at low doses routinely used in clinical practice has not been systematically evaluated. We compared the heart rate-corrected QT interval using the subject-specific correction to results obtained by Bazett's and Fridericia's corrections. Our objective was to determine if different heart rate correction methods lead to different conclusions about the ability of a drug to prolong the QT interval.
Methods
Subjects and study design
We studied 16 nonsmoking healthy volunteers aged between 21 and 41 years. The study was conducted in Georgetown University's General Clinical Research Center (GCRC). All volunteers gave written informed consent prior to participation in the study. The study protocol was approved by the Institutional Review Board of Georgetown University Medical Center. This protocol was a randomized, double-blind, placebo-controlled crossover clinical trial. There were two study periods with each period lasting 4 days. There was a 3-day washout between the two study periods. Subjects were randomly assigned to receive either a single placebo capsule or a single haloperidol tablet (Geneva Pharmaceuticals Lot no. 114748; Broomfield, CO, USA) at 08.00 h during the first study period and the alternative treatment at the identical time during the second study period. Electrocardiograms (ECGs) were collected before and 2, 4, 6, 8, 10, 24, 36, 48, 60, 72, 84, and 96 h after administration of either placebo or haloperidol. A total of 13 recordings were performed in the placebo period and 13 recordings performed in the treatment period. ECGs were obtained with the subject resting supine for approximately 15 min prior to acquisition using a MAC 5000 ECG machine (GE Medical Systems, Milwaukee, WI, USA). We acquired 12-lead ECGs at a paper speed of 50 mm s−1 and an amplitude of 20 mm mV−1. The electrocardiograms were printed in a format that displayed rhythm strips from all 12 leads simultaneously in order to facilitate identification of the earliest Q wave and latest T wave observable. Details of the subjects studied and study design have been described elsewhere [17].
QT interval analysis
A trained physician blinded to the study protocol and sequence of the ECGs used a previously described and validated computer–operator interactive method to measure QT intervals [18]. The ECG tracings were placed on a digitizing pad (Summagraphics®, Hacienda Heights, CA, USA) that was connected to a personal computer. A cross-hair pointing device was used to mark the beginning and end of the QT interval as well as the preceding RR interval among three consecutive beats in which the RR interval was relatively constant. The average of the three consecutive RR and QT intervals was used. The QT interval was corrected for heart rate variability using a simplified version of the approach described by Malik [12]. This method involved plotting the QT interval vs RR interval relationship derived from each subject's placebo period, and fitting the data to a linear mixed model of the form logQTij = Bi + αi logRRij + eij, where eBi was the subject-specific QT in seconds when the RR interval was 1 s, αi was the slope of the log-transformed RR vs QT relationship, and eij was an error term. The subscripts i and j refer to an individual subject i recorded at a study time point j. A mathematical manipulation of the linear model described, yielded a correction of the form: Qtcij=Qtij/(RRij)αi. The correction for each individual derived from placebo period data was applied to the treatment period data.
Heart rate corrections of the QT interval were also performed using Bazett's formula QTcB = QT/√RR and Fridericia's formula QTcF = QT/3√RR, where both the QT and RR intervals are in seconds.
Statistical and power analysis
A paired t-test was used to compare the mean intersubject QTc on treatment at a given time point vs the mean intersubject QTc on placebo at the corresponding time point. Because 13 t-test comparisons were made inclusive of the baseline and 96-h time points, multiplying the raw P-value by 13 performed a Bonferroni correction for multiple comparisons. Changes in heart rate were analysed in a similar way. P-values < 0.05 were considered statistically significant.
Slope comparisons of the QTcvs RR linear regression lines for the three heart rate correction formulae (Bazett, Fridericia, and subject specific) were performed using ai paired two-tailed t-test.
Given a sample size of 16 subjects in a crossover trial, there was 80% power to detect a difference between the haloperidol and placebo-treated groups of 13 ms or greater at an α level of 5%. This was based on the assumption that the intersubject standard deviation of the QTc (Bazett's correction) was approximately 18 ms [19].
Results
Sixteen healthy adult volunteers (eight males, eight females) participated in the study. The body mass index (BMI) ranged from 21.4 to 31.6 kg m−2 in all volunteers. The mean male BMI was 25.8 kg m−2 (95% confidence interval [CI] 23.1, 28.5) and was statistically significantly (P = 0.03) different from the mean female BMI of 22.4 kg m−2 (95% CI 21.3, 23.5). At 10 h post-dosing, the mean heart rate on haloperidol was 62.3 beats min−1 (95% CI 58.8, 65.8) and was statistically significantly (P = 0.039, post-Bonferroni) greater than the mean heart rate on placebo of 56.3 beats min−1 (95% CI 51.9, 60.7). The heart rate was not statistically significantly different between the two groups at any other time point during the study.
Using linear mixed modelling for QT correction, the mean α ± SD for our study sample was 0.29 (95% CI 0.27, 0.31), where α was the slope of the log-transformed QT vs RR relationship. The terms corresponding to α in Bazett's and Fridericia's heart rate correction formulae were constant at 1/2 and 1/3, respectively. The mean correction term, α, in men was 0.31 (95% CI 0.28, 0.34) and was statistically significantly (P = 0.04) higher than the mean correction term in women of 0.27 (95% CI 0.25, 0.29) as shown in Figure 1.
Figure 1.
Individual correction term, αi for the log-transformed QT vs RR relationship for all study subjects (n = 16) and males (n = 8) and females (n = 8) separately.
As expected, there was a positive sloping linear regression line between the uncorrected QT interval and RR interval using composite data from all study subjects (Figure 2A). As shown in Figure 2B, there was a negative sloping linear regression between Bazett's heart rate-corrected QT (QTcB) and the RR interval. At faster heart rates, the QTcB was over-corrected while at slower heart rates the QTcB was under-corrected when the composite data from all study subjects were used. The linear regression of the QTcvs RR relationship derived from Fridericia's heart rate-corrected QT (QTcF) was more independent of the RR interval than was the linear regression of the data obtained with QTcB (Figure 2C). The linear regression line of QTcSS was similar in slope to the linear regression of QTcF (Figure 2D). However, upon comparing the absolute slopes of the QTcvs RR linear regression for each individual, using the three heart rate correction formulae described, the subject-specific correction proved to be the superior correction (i.e. to have the lowest value of the slope of the RR/QTc relationship). The goal of heart rate correction of the QT interval was to obtain a QTcvs RR linear regression line with a slope as close to zero as possible in each study subject. The mean absolute slope of the QTc interval (ms) vs RR interval (ms) regression line from placebo period data using each individual's subject-specific correction was 0.02 (95% CI 0.01, 0.03). The mean subject-specific slope was statistically significantly (P = 0.01) lower than the mean absolute (QTcvs RR regression line) slope of 0.043 (95% CI 0.03, 0.06) obtained using Fridericia's correction. The mean subject-specific slope was also statistically significantly (P < 0.0001) lower than the mean absolute slope (QTcvs RR regression line) obtained using Bazett's correction of 0.10 (95% CI 0.07, 0.13).
Figure 2.
A composite uncorrected QT vs RR interval relationship and heart rate-corrected QT vs RR relationship using Bazett’s, Fridericia’s, and subject-specific methods (n = 16). (a) Uncorrected QT interval (ms) vs RR interval (ms). (b) Heart rate-corrected QT using Bazett's method (QTcB) vs RR. (c) Heart rate-corrected QT using Fridericia's method (QTcF) vs RR. (d) Heart rate-corrected QT using the subject-specific method (QTcSS) vs RR. Note the QT vs RR relationships were taken from placebo period data.
At 10 h post-haloperidol administration the mean QTc on haloperidol was 425.4 ms (95% CI 414.3, 436.5) and was statistically significantly (P = 1.7 × 10−5 post-Bonferroni) greater than the mean QTc on placebo of 403.1 ms (95% CI 394.3, 411.9) using Bazett's correction. Similarly, using Fridericia's correction, the mean QTc on haloperidol 10 h post-drug administration was 423.1 ms (95% CI 412.6, 433.6) and was statistically significantly (P = 0.00077 post-Bonferroni) greater than the mean QTc on placebo of 408.2 ms (95% CI 398.6, 417.8). Using the subject-specific correction, at 10 h post-haloperidol administration, the mean QTc on haloperidol was 421.6 ms (95% CI 410.8, 432.4) and was statistically significantly (P = 0.0053 post-Bonferroni) greater than the mean QTc on placebo of 408.4 ms (95% CI 398.6, 417.8). Beside the 10-h time point, no other time points produced statistically significant results using any of the three correction methods. Figure 3(A-C) shows the mean QTc changes during the study as defined by the mean QTc on treatment at a particular time point minus the QTc on placebo at the corresponding time point.
Figure 3.
Mean heart rate-corrected QT (QTc) change ± SEM as a function of time post-haloperidol administration using (a) Bazett's method (QTcB), (b) Fridericia's method (QTcF), and (c) subject-specific method (QTcSS). The QTc change is defined as the QTc (treatment) − QTc (placebo) obtained from electrocardiograms at identical time points post-haloperidol and post-placebo. *The mean QTc on treatment was statistically significantly greater on haloperidol than placebo with a P < 0.05.
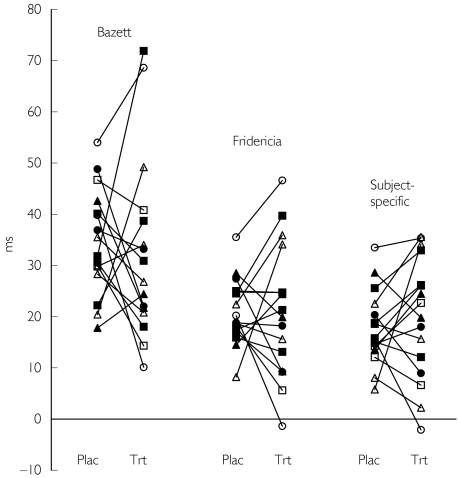
The distribution of QTc changes from baseline in increments of 10 ms is displayed in Table 1 using the three correction methods. The graphical representation of QTc changes from baseline is displayed in Figure 4. The interindividual range of maximal QTc changes from baseline was broadest using Bazett's method (10 ms to 73 ms), intermediate with Fridericia's method (−3 ms to 48 ms), and smallest with the subject-specific correction (−3 ms to 38 ms). Regardless of method, the ‘on treatment’ interindividual maximal QTc changes were always greater than ‘on placebo’ changes.
Table 1.
Distribution of QTc changes from baseline in increments of 10 ms using the three correction methods (n = 16).
| Bazett | Fridericia | Subject-specific | ||||
|---|---|---|---|---|---|---|
| Ms | Treatment | Placebo | Treatment | Placebo | Treatment | Placebo |
| < 0 | 0 | 0 | 1 | 0 | 1 | 0 |
| ≥ 0 but < 10 | 0 | 0 | 3 | 1 | 3 | 2 |
| ≥ 10 but < 20 | 3 | 1 | 4 | 7 | 4 | 9 |
| ≥ 20 but < 30 | 5 | 4 | 4 | 7 | 4 | 4 |
| ≥ 30 but < 40 | 4 | 6 | 3 | 1 | 4 | 1 |
| ≥ 40 | 4 | 5 | 1 | 0 | 0 | 0 |
Figure 4.
Maximal individual QTc changes from baseline (t = 0) occurring on placebo (plac) and on haloperidol (trt) using the correction methods of Bazett, Fridericia, and the subject-specific method.
Discussion
We studied the QT prolonging effects of single oral doses of haloperidol in a randomized, blinded, crossover trial. We found statistically significant mean QTc prolongations of 13–22 ms relative to placebo occurring 10 h post-haloperidol administration depending on the correction method used. Although the magnitude of mean QTc prolongation varied between the three methods, the conclusion that single doses of oral haloperidol can prolong the QTc at low doses was consistent.
Bazett's correction tended to produce maximal QTc changes from baseline during either placebo or treatment periods that were generally greater than those derived using the subject-specific method. In addition, there were two outliers (subjects with QTc ≥ 60 ms) on haloperidol using Bazett's correction, whereas no outliers were present using the subject-specific method. The reason for this variability was that the correction term of Bazett (0.5) was completely outside the range of correction terms derived using the subject-specific correction (0.23–0.38). Our findings are consistent with another study in which the range of individual values was 0.234–0.486 [11]. This confirms the notion that Bazett's formula should not be used in drug studies because it may not only generate a false signal when heart rate increases on treatment, but also mask an existing signal when heart rate decreases on treatment.
The maximal QTc changes from baseline were similar when comparing Fridericia's method and the subject-specific method, because the correction term used in the former method (1/3) was inside the range of values derived using the latter method. However, there were some inconsistencies between the two methods in certain individuals. For example, one individual had a QTc change from baseline of 35.5 ms on placebo and 46.6 ms on haloperidol using Fridericia's method. The same individual had changes from baseline of 33.5 ms and 35.3 ms, respectively, using the subject-specific method. The QTc changes from baseline on haloperidol comparing Fridericia's method and the subject-specific method varied by about 11 ms. While this magnitude of change may not be clinically meaningful in a single individual, potential exists for much larger QTc variations, particularly when studying a drug that changes heart rate much more than the mean heart rate change of 6 beats min−1 associated with haloperidol. Also it is possible that inconsistencies in Fridericia's correction may change the number of outliers (e.g. subjects with QTc ≥ 60 ms). Finally, mean changes of this magnitude in larger groups of subjects can have important implications to drug regulators and developers.
Our results conflict with another study in the literature in which the QT-prolonging effects of low doses of haloperidol were reported in a population with Tourette's syndrome [20]. In that study, a nonstatistically significant QT prolongation of approximately 4 ms was found. However, there were major differences in the two study designs. While our study was a single-dose study in 16 healthy subjects, the study in Tourette's patients was a multidose study involving 42 subjects with a mean orally administered dose of 5 mg (range 0–10 mg). We acknowledge the limitations of extrapolating results from our single-dose study in healthy subjects to a study of multiple doses in a patient population (e.g. with Tourette's or schizophrenia). However, we felt our crossover study design provided greater power, because unlike the Tourette's study, all subjects who got haloperidol also got placebo. Additionally, because we acquired ECGs after a 15-min resting period, we had markedly lower standard deviations of 18.5 ms and 19.9 ms (using Bazett's correction) on treatment and placebo, respectively, than did the Tourette's study, where corresponding values were 28.0 ms and 22.0 ms. It is unclear whether resting ECGs were acquired in the Tourette's study. Finally, we acquired multiple ECGs at prespecified time-points post-drug administration that allowed us to capture a ‘peak’ QT effect that was delayed relative to dosing. It is unclear in the Tourette's study when ECGs were acquired relative to dosing. We cannot confirm that our results represent a false positive without concomitant administration of a negative control. QT studies involving concomitant negative controls are not commonly done and are less important than studies involving a positive control, because we care less about drugs that do not cause QT prolongation. In general, we are more willing to tolerate a false-positive QT effect of a drug rather than a false-negative effect.
Our study also showed that the subject-specific heart rate correction method using linear mixed modelling was superior to standardized heart rate correction methods of Bazett and Fridericia based on producing QTcvs RR regression lines that were individually independent of heart rate (RR interval). However, as we used only a simplified version of the individual QT/RR assessment, the subject-specific correction used in the study was not absolutely perfect. We derived the subject-specific correction from a total of 13 ECGs obtained in the placebo period. We could have improved the results of the model by increasing the number of ECGs obtained in each subject during the placebo period. Malik acquired over 40 ECGs in each subject's placebo period data in generating a subject-specific correction [12]. We could also have improved our model by acquiring ECGs over a broader range of heart rates to obtain the best possible fit of the QT vs RR data. A frequently suggested way of obtaining a broad range of heart rates is through exercise testing. However, the complexity with exercise testing is that it takes substantial time for the QT interval to adapt to changes in the RR interval [21]. After a sudden, sustained change in heart rate, it can take 2–3 min for a new equilibrium to be reached between the action potential duration and the new RR interval. Also, it is not known how the physiological changes, namely the marked sympathetic overdrive, accompanying exercise can affect the nature of the QT vs RR relationship.
An assumption of the subject-specific correction is that the QT vs RR relationship and the correction term α (slope term) remain constant over a period of days to weeks in any given individual. We derived our subject-specific model from placebo period data and applied this model to the treatment period data that were separated by a 1-week period from the placebo period. We believe our assumption is justified because investigators have shown that this relationship is preserved within an individual over at least 1 month [22].
In addition to displaying significant interindividual variability, our results showed that the QT vs RR relationship was also different between men and women in general. The implications of this are important when considering a population-based correction that could potentially be used in larger phase 2/3 clinical trials rather than a subject-specific correction that would primarily be used in smaller phase I trials. When performing a population-based correction, it may be valuable to derive separate heart rate corrections of QT in males and females.
This study demonstrates a role for using the subject-specific correction method to minimize potential for over- or under-correction of the QTc, particularly in phase I clinical pharmacology studies where dense ECG sampling is possible. Although potential exists for contrasting conclusions depending on the method of heart rate correction used, as evidenced by Malik's work, we did not arrive at such a conclusion. However, differences in the magnitude of mean QTc prolongation and interindividual QTc changes from baseline depending on correction method used is noteworthy. In doing such types of phase I QT studies, the roles of appropriate positive and in some instances negative controls can not be overlooked.
Acknowledgments
Supported in parts by grants T32-9M 08386 and RO1-GM56898-01 from the National Institutes of General Medical Sciences, Bethesda, MD, and by the Georgetown University GCRC (P. I. Raymond L. Woosley MD, PhD). Data from this study are deposited in the Pharmacogenetics Knowledge Base (pharmgkb.org) supported by U01-GM61374.
References
- 1.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 2.Fridericia LS. Die Systolendauer im Elektrokardiogramm bei normalen Menchan und bei Herzdranken. Acta Med Scand. 2002;53:469–486. [Google Scholar]
- 3.Malik M. The imprecision in heart rate correction may lead to artificial observations of drug induced QT interval changes. Pacing Clin Electrophysiol. 2002;25:209–216. doi: 10.1046/j.1460-9592.2002.00209.x. [DOI] [PubMed] [Google Scholar]
- 4.Benatar A, Decraene T. Comparison of formulae for heart rate correction of QT interval in exercise ECGs from healthy children. Heart. 2001;86:199–202. doi: 10.1136/heart.86.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glassman AH, Bigger JT., Jr Antipsychotic drugs: prolonged QTc interval, torsade de pointes, and sudden death. Am J Psychiatry. 2001;158:1774–1782. doi: 10.1176/appi.ajp.158.11.1774. [DOI] [PubMed] [Google Scholar]
- 6.Awni WM, Cavanaugh JH, Leese P, et al. The pharmacokinetic and pharmacodynamic interaction between zileuton and terfendine. Eur J Clin Pharmacol. 2002;52:49–52. doi: 10.1007/s002280050248. [DOI] [PubMed] [Google Scholar]
- 7.Hodges M, Salerno D, Erlien D. Bazett's QT correction reviewed: evidence that a linear QT correction for heart rate is better. J Am Coll Cardiol. 1983;1:694. [Google Scholar]
- 8.Milne JR, Camm AJ, Ward DE, Spurrell RA. Effect of intravenous propranolol on QT interval. A new method of assessment. Br Heart J. 1980;43:1–6. doi: 10.1136/hrt.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Browne KF, Prystowsky E, Heger JJ, Chilson DA, Zipes DP. Prolongation of the Q–T interval in man during sleep. Am J Cardiol. 2002;52:55–59. doi: 10.1016/0002-9149(83)90068-1. [DOI] [PubMed] [Google Scholar]
- 10.Mayuga KA, Parker M, Sukthanker ND, Perlowski A, Schwartz JB, Kadish AH. Effects of age and gender on the QT response to exercise. Am J Cardiol. 2001;87:163–167. doi: 10.1016/s0002-9149(00)01309-6. [DOI] [PubMed] [Google Scholar]
- 11.Malik M, Farbom P, Batchvarov V, Hnatkova K, Camm AJ. Relation between QT and RR intervals is highly individual among healthy subjects: implications for heart rate correction of the QT interval. Heart. 2002;87:220–228. doi: 10.1136/heart.87.3.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malik M. Problems of heart rate correction in assessment of drug-induced QT interval prolongation. J Cardiovasc Electrophysiol. 2001;12:411–420. doi: 10.1046/j.1540-8167.2001.00411.x. [DOI] [PubMed] [Google Scholar]
- 13.Suessbrich H, Schonherr R, Heinemann SH, Attali B, Lang F, Busch AE. The inhibitory effect of the antipsychotic drug haloperidol on HERG potassium channels expressed in Xenopus oocytes. Br J Pharmacol. 1997;120:968–974. doi: 10.1038/sj.bjp.0700989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bett JH, Holt GW. Malignant ventricular tachyarrhythmia and haloperidol. BMJ. 1983;287:1264. [Google Scholar]
- 15.Menes C, Burra P, Hoaken PC. Untoward effects following combined neuroleptic-lithium therapy. Can J Psychiatry. 1980;25:573–576. doi: 10.1177/070674378002500707. [DOI] [PubMed] [Google Scholar]
- 16.Sharma ND, Rosman HS, Padhi D, Tisdale JE. Torsades de Pointes associated with intravenous haloperidol in critically ill patients. Am J Cardiol. 1998;81:238–240. doi: 10.1016/s0002-9149(97)00888-6. [DOI] [PubMed] [Google Scholar]
- 17.Desai M, Tanus-Santos JE, Desta S, et al. TPII-47/low doses of oral haloperidol do not cause QT prolongation in poor metabolizers of CYP2D6. Am Soc Clin Pharmacol Therapeutics. 2002;71:53. [Google Scholar]
- 18.Woosley RL, Sale M. QT interval: a measure of drug action. Am J Cardiol. 1993;72:36B–43B. doi: 10.1016/0002-9149(93)90039-f. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez I, Kilborn MJ, Liu XK, Pezzullo JC, Woosley RL. Drug-induced QT prolongation in women during the menstrual cycle. JAMA. 2001;285:1322–1326. doi: 10.1001/jama.285.10.1322. [DOI] [PubMed] [Google Scholar]
- 20.Fulop G, Phillips RA, Shapiro AK, Gomes JA, Shapiro E, Nordlie MA. ECG changes during haloperidol and pimozide treatment of Tourette's disorder. Am J Psychiatry. 1987;144:673–675. doi: 10.1176/ajp.144.5.673. [DOI] [PubMed] [Google Scholar]
- 21.Franz MR, Swerdlow CD, Liem LB, Schaefer J. Cycle length dependence of human action potential duration in vivo. Effects of single extrastimuli, sudden sustained rate acceleration and deceleration, and different steady-state frequencies. J Clin Invest. 1988;82:972–979. doi: 10.1172/JCI113706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Batchvarov V, Ghuran A, Dilaveris P, et al. QT–RR relationship in healthy subjects exhibits substantial intersubject variability and high intrasubject stability. Am J Physiol Heart Circ Physiol. 2002;282:H2356–H2363. doi: 10.1152/ajpheart.00860.2001. [DOI] [PubMed] [Google Scholar]