Abstract
Aims
To develop a polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP)-based assay to genotype for hepatic CYP3A5 expression and to use this assay to study a British population.
Methods
CYP3A5-specific PCR primers were designed with one including a base-pair mismatch to create a RsaI site in samples positive for A6986 (CYP3A5*1 allele). Following PCR and RsaI digestion, different band patterns on electrophoresis were predicted for individuals positive for CYP3A5 (CYP3A5*1 allele) compared with those who do not express the gene (CYP3A5*3 homozygotes). The assay was validated by DNA sequencing. DNA samples from a human liver bank consisting of 22 livers whose CYP3A5 expression had been determined by immunoblotting and a group of random individuals (n = 100) from the North-east of England were genotyped by the new assay.
Results
In the liver bank, five out of 22 samples expressed CYP3A5 at significant levels (>20 pmol mg−1 protein) and were found to have the genotype CYP3A5*1/CYP3A5*3 by the PCR-RFLP assay. All other liver DNA samples were CYP3A5*3 homozygotes. In the group of 100 random individuals, 13 had the genotype CYP3A5*1/CYP3A5*3 and all others were CYP3A5*3 homozygotes, predicting that 13% (95% confidence interval (CI) 6%, 20%) would show significant hepatic CYP3A5 expression. The frequency for the CYP3A5*1 allele was 0.065 (95% CI 0.032, 0.097).
Conclusions
We have developed a simple assay for the detection of the CYP3A5*1/CYP3A5*3 alleles and shown that in a British population their frequency is similar to that reported previously. We have also shown a good correlation between hepatic CYP3A5 expression and genotype for a British Caucasian liver bank.
Keywords: CYP3A5, cytochrome P450, human liver, PCR assay, pharmacogenetics
Introduction
Many drugs in common therapeutic use are metabolized by members of the cytochrome P450 CYP3A subfamily [1], in which there is considerable interindividual variation in gene expression. The 231-kb CYP3A locus includes the CYP3A4, CYP3A5, CYP3A7 and the recently discovered CYP3A43 genes [2]. CYP3A5 is expressed in only 10–30% of adult human livers [3, 4]. Several single nucleotide polymorphisms (SNPs) have recently been found in CYP3A5 including A6986G in intron 3, which is the CYP3A5*3 allele [5–7]. Carriers of CYP3A5*3 appear not to express the enzyme due to the creation of a cryptic splice site. This results in the incorporation of an intron sequence in the mature mRNA, and the production of a truncated protein due to premature termination of translation [5]. The CYP3A5*1 allele with A at position 6986 appears to be the main allele associated with CYP3A5 expression and activity. When expressed, CYP3A5 may constitute up to 50% of total hepatic CYP3A protein [5]. Individuals with a CYP3A5*1 allele may show more rapid than average metabolism of CYP3A substrates, since a number of CYP3A4 substrates including erythromycin and midazolam are also efficiently metabolized by CYP3A5 [8, 9]. However, there are also many compounds which are less efficiently metabolized by CYP3A5 than CYP3A4 (e.g. aflatoxin B1, quinidine, testosterone, irinotecan) and a few examples where CYP3A5 is the more efficient oxidizing enzyme (e.g. all-trans retinoic acid) [8–12]. As CYP3A5 expression in the liver is likely to be a useful predictor of metabolism of a variety of drugs, we have developed a simple polymerase chain reaction (PCR)-restriction fragment length polymorphism (RFLP)-based assay to genotype for the CYP3A5*1 and CYP3A5*3 alleles. By genotyping DNA samples from a British human liver bank and detecting CYP3A5 expression by immunoblotting, we have shown that CYP3A5 genotype correlates with phenotype in this UK-based subject group. We also determined the frequency of these CYP3A5 alleles in a group of British volunteers.
Materials and methods
Subjects
The volunteer group consisted of 100 individuals (58 male, 42 female) aged 38–91 years (median 69 years) recruited from the registers of GP practices in North-east England. The study was approved by the Newcastle University and Newcastle & North Tyneside joint ethics committee and all volunteers gave informed consent for their blood samples to be used in studies on cytochrome P450 polymorphisms. The liver bank consisted of 22 British Caucasian samples obtained from University of Aberdeen with the approval of the Grampian Health Board ethics committee.
Preparation of DNA and human liver microsomes
DNA was extracted from blood samples using the method described by Daly et al. [13]. DNA was prepared from liver by proteinase K digestion followed by phenol-chloroform extraction using standard methods [14]. Microsomes were prepared by differential centrifugation as described previously [15].
Genotyping for CYP3A5
PCR amplification was performed using the primers 3A5A (5′-CCTGCCTTCAATTTTCACT-3′) and 3A5B (5′-GGTCCAAACAGGGAAGAGGT-3′). Primer design used sequence data for the CYP3A5*1 and CYP3A5*3 alleles from GenBank (accession numbers J04813 and AC005020, respectively). A single mismatch was introduced into the penultimate position of the reverse primer (see underlined G in primer 3A5B) to create a RsaI restriction site when A is present at the adjacent position 6986 (CYP3A5*1 allele). PCR was performed in a final volume of 25 µl with 0.25 µm each primer, 0.1 mm each dNTP, 0.2–0.5 µg genomic DNA, 0.25 U Taq polymerase (Promega, Madison, WI, USA) in 50 mm Tris-HCl pH 9.1, 3.5 mm MgCl2, 16 mm ammonium sulphate, 0.15 mg ml−1 bovine serum albumin. Amplification involved 35 cycles of 94°C for 1 min, 61°C for 1 min and 70°C for 1 min followed by final extension at 72°C for 7 min. A product of 196 bp was obtained. For restriction digest of the PCR product, a 20-µl aliquot was incubated with 3 U RsaI (New England Biolabs, Hitchin, Herts, UK) overnight at 37°C. Digestion products were analysed by electrophoresis on a 10% polyacrylamide gel (170 × 200 × 1 mm) at 150 V (constant voltage) for 6 h using 1 × TBE (0.09 m Tris, 0.09 m boric acid, 2.5 mm EDTA pH 8.3) as the electrophoresis buffer. Gels were stained with ethidium bromide for 15 min and band patterns visualized using a u.v. transilluminator.
DNA sequencing
To purify PCR products for DNA sequencing, approx. 300 µl PCR product was mixed with 700 ml binding solution (0.1% (w/v) diatomaceous earth in 8 m guanidine HCl). The suspension was transferred to a mini spin filter in a microcentrifuge tube and centrifuged at 3000 g for 2 min. The filtrate at the bottom of the tube was discarded and the filter replaced in the same tube. Wash buffer (600 µl; 200 mm NaCl, 10 mm EDTA, 50 mm Tris-HCl pH 7.4 in 50% (v/v) ethanol) was added and the pellet was washed by centrifugation at 5000 g for 2 min. This wash step was repeated followed by a final centrifugation for 2 min at 5000 g to remove all traces of wash. Finally, the filter was transferred to a 1.5-ml collection tube and the bound DNA was eluted in 35 µl water by centrifugation at 10 000 g for 5 min. Approx. 100–500 ng DNA was sequenced using fluorescently labelled dye terminators (MWG Biotech., Milton Keynes, UK).
Immunoblotting
Human liver microsomal proteins (up to 20 µg per lane) were size fractionated by electrophoresis on a 10% (w/v) SDS-polyacrylamide gel [16]. Electrophoretic transfer of protein from the gel to Hybond-C Extra nitrocellulose membrane (Amersham-Pharmacia, Amersham, Bucks, UK) was carried out using a Transblot cell (BioRad, Hemel Hempstead, Herts, UK) for 4 h at 60 V and 4°C. The filter was blocked in 0.14 m NaCl, 25 mm Tris-HCl, pH 7.4 containing 10% dried milk and 0.5% (v/v) Tween 20 for at least 1 h and then incubated with an anti-CYP3A5 serum (1 : 3000) (Gentest, Woburn, MA, USA) or anti-CYP3A4 serum (1 : 750) (Gentest), followed by washing and incubation with peroxidase-conjugated goat antirabbit serum. After washing, peroxidase activity was detected by ECL detection (Amersham). CYP3A4/5 expression was quantified by densitometry using Kodak Digital Science image analysis software with a standard curve for CYP3A4 (range 140–560 fmol lane−1) or CYP3A5 supersomes (Gentest) (range 110–880 fmol lane−1) as appropriate included on each gel. The limit of detection for both CYP3A4 and CYP3A5 detection was 1 fmol µg−1 protein at a maximum loading of 20 µg protein.
Statistical analysis
Population genotype frequencies were compared using Fisher's exact test. For proportions, 95% confidence intervals were calculated.
Results
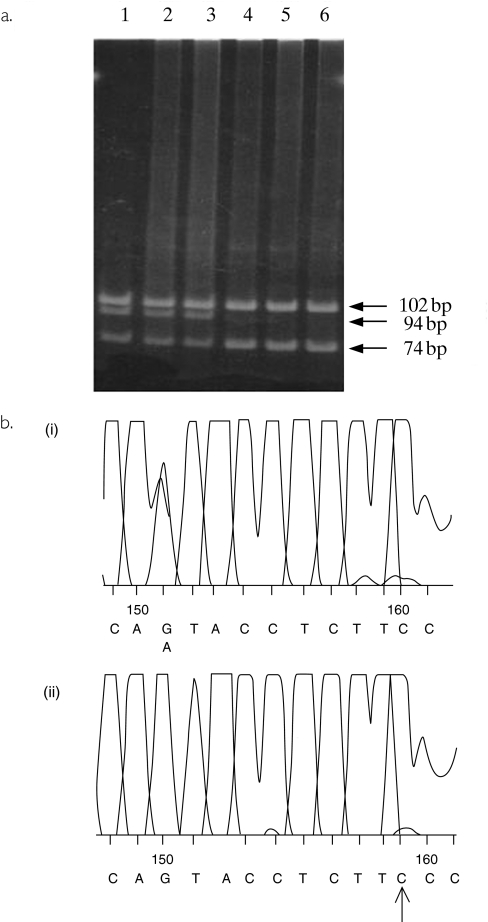
Though the A6986G polymorphism has been identified by DNA sequencing in two published studies [5, 6] and appears to be the main polymorphism associated with absence of CYP3A5 expression in the liver, no simple PCR-based assay suitable for routine genotyping has so far been described. The base substitution is not associated with a change in restriction digest pattern, but, by including a single base-pair mismatch in the reverse primer, it was possible to introduce a restriction site into PCR products positive for A at position 6986. As shown in Figure 1a, following PCR-RFLP analysis, samples heterozygous for CYP3A5*1 showed four bands of 102 bp, 94 bp, 74 bp and 20 bp, whereas those homozygous for CYP3A5*3 showed two bands of 102 bp and 94 bp. Samples homozygous for CYP3A5*1 are predicted to show three bands of 102 bp, 74 bp and 20 bp, but no samples with this genotype were detected in the present study. The assay was validated by sequencing samples from two subjects heterozygous for CYP3A5*1 and from two subjects homozygous for CYP3A5*3. As summarized in Figure 1b, subjects homozygous for CYP3A5*3 had a G only present at the expected position whereas for CYP3A5*1 heterozygotes, peaks for A and G were superimposed.
Figure 1.
(a) Polyacrylamide gel electrophoresis of digested PCR products. Lanes 1–3 show band patterns for samples with a CYP3A5*1/CYP3A5*3 genotype. Lanes 4–6 show band patterns for samples from subjects with a CYP3A5*3/CYP3A5*3 genotype. (b) Sequencing traces of PCR products from samples positive and negative for CYP3A5*1. Panel (i) shows a trace from a sample from a subject with the genotype CYP3A5*1/CYP3A5*3 and panel (ii) a CYP3A5*3/CYP3A5*3 genotype. The mismatched primer has resulted in the introduction of C in place of T in the forward sequence which is indicated by an arrow.
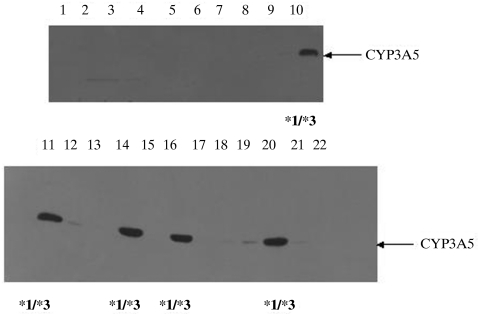
The human liver DNA samples were genotyped by the newly developed assay and five of these samples (23%; 95% confidence interval (CI) 5%, 41%) were found to be heterozygous for the A6986G polymorphism with the other 16 samples all homozygous for G at this position. Expression of CYP3A5 protein was detected in eight of the 22 samples. However, expression in three of these samples was weak and did not exceed 4 pmol mg−1 protein (range 1.25–3.6 pmol mg−1). Five samples contained approximately ten times more CYP3A5 than this, in the range 29–66 pmol mg−1 protein (median 37 pmol mg−1). As summarized in Figure 2, these five samples were genotyped as being heterozygous for the CYP3A5*3 allele. The median CYP3A4 content of all livers was 33 pmol mg−1 protein (range < 1–93 pmol mg−1 protein) compared with 37 pmol mg−1 (range 29–66 pmol mg−1) for the five livers expressing significant CYP3A5. This indicates that in CYP3A5 expressors approximately 50% of all hepatic CYP3A may be CYP3A5 as suggested previously [5, 17]. There was no indication that livers that failed to express detectable CYP3A5 showed significantly higher or lower CYP3A4 expression (median 31 pmol mg−1 protein; range < 1–93 pmol mg−1 protein).
Figure 2.
Immunoblot of human liver microsomes using anti-CYP3A5 antibody as the primary antiserum. The samples in lanes 10, 11, 14, 16 and 20 showed strong positive bands which comigrated with a CYP3A5 standard. As indicated, each of these samples had the genotype CYP3A5*1/CYP3A5*3.
To determine CYP3A5 genotype and allele frequencies in a larger British population, genotyping was performed on 100 DNA samples from apparently healthy Caucasians resident in North-east England. Thirteen of these subjects were found to be heterozygous for the CYP3A5*3 allele (13%; 95% CI 6%, 20%) with the remainder (87%; 95% CI 80%, 94%) being homozygous for this allele. Frequencies of the two alleles in this population were therefore 0.06 ± 0.03 for CYP3A5*1 and 0.94 ± 0.03 for CYP3A5*3. There was no significant difference in allele frequencies between this population and those reported in two previous studies of Caucasians [6, 17].
Discussion
The wide interindividual variation in CYP3A isoform expression and functional activity is well established, and is thought to be caused by both genetic and environmental factors [18, 19]. A number of genetic polymorphisms have now been described for CYP3A4, the CYP3A isoform found at the highest concentration in most livers. However, these polymorphisms are rare, especially among Caucasians, and generally of limited functional significance [6, 20, 21]. Therefore, it has been suggested that polymorphisms affecting other genes, particularly transcription factors, may determine individual CYP3A4 expression. However, apart from some recent reports of polymorphisms in the major CYP3A4 regulator PXR, none has yet been identified [22, 23]. For many CYP3A4 drug substrates, there is no clear information on the contribution of CYP3A5 to their metabolism [17], although a recent study estimated the contribution of CYP3A4, CYP3A5 and CYP3A7 to the metabolism of ten drug substrates [9]. It is clear from both previous studies and the current work that when CYP3A5 is expressed at 20 pmol mg−1 protein or higher, it represents a significant proportion of the total hepatic CYP3A content. Although CYP3A4 and CYP3A5 (when expressed) are the major contributors to hepatic CYP3A content, approximately 10% of adult livers also express CYP3A7 mRNA, but it is unclear whether protein is expressed [5]. The recently discovered CYP3A43 enzyme is not believed to make a significant contribution to hepatic CYP3A activity [2, 24].
In the present study, five liver samples showed clear CYP3A5 expression in excess of 20 pmol mg−1 protein and were also positive for CYP3A5*1. Three samples showed low but detectable CYP3A5 expression and were negative for CYP3A5*1. The basis of the absence of hepatic CYP3A5 expression is the introduction of a new splice site when G is present at position 6986 in the CYP3A5*3 allele. However, in this case, correct RNA splicing may also be occurring at a lower rate, leading to the production of a low concentration of active enzyme. This finding is in line with previous observations for both liver and small intestine [5]. The five CYP3A5*1-positive livers showed less variation in CYP3A5 expression than that observed for CYP3A4. This may be due to CYP3A5 not being inducible, via either PXR or other possible regulators such as CAR-β[5, 19].
In addition to the A6986G polymorphism, it has been suggested that other variant alleles may lead to an absence of CYP3A5 expression [5, 6]. However, our screening of the liver bank indicates that these are likely to be of minor importance in UK Caucasians.
Acknowledgments
We are grateful to Professor GM Hawksworth, University of Aberdeen for supplying human liver samples. B.P.K. is supported by a studentship from Genotype Ltd.
References
- 1.Guengerich FP. Cytochrome P450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Gellner K, Eiselt R, Hustert E, et al. Genomic organization of the human CYP3A locus: identification of a new, inducible CYP3A gene. Pharmacogenetics. 2001;11:111–121. doi: 10.1097/00008571-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama T, Yamano S, Waxman DJ, et al. Cytochrome P450 hPCN3, a novel cytochrome P450 IIIA gene product that is differentially expressed in adult human liver. J Biol Chem. 1989;264:10388–10395. [PubMed] [Google Scholar]
- 4.Wrighton SA, Brian WR, Sari MA, et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (Hlp3) Mol Pharmacol. 1990;38:207–213. [PubMed] [Google Scholar]
- 5.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nature Genet. 2001;27:383–391. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 6.Hustert E, Haberl M, Burk O, et al. The genetic determinants of the CYP3A5 polymorphism. Pharmacogenetics. 2001;11:773–779. doi: 10.1097/00008571-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 7. http://;www.imm.ki.se/CYPalleles/
- 8.Gillam EMJ, Guo Z, Ueng Y-F, et al. Expression of cytochrome P450 3A5 in Escherichia coli: effects of 5′modification, purification, reconstitution conditions, and catalytic activities. Arch Biochem Biophys. 1995;317:374–384. doi: 10.1006/abbi.1995.1177. [DOI] [PubMed] [Google Scholar]
- 9.Williams JA, Ring BJ, Cantrell VE, et al. Comparative metabolic capabilities of CYP3A4, CYP3A5 and CYP3A7. Drug Metab Dispos. 2002;30:883–891. doi: 10.1124/dmd.30.8.883. [DOI] [PubMed] [Google Scholar]
- 10.Santos A, Zanetta S, Cresteil T, et al. Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans. Clin Cancer Res. 2000;6:2012–2020. [PubMed] [Google Scholar]
- 11.Nielsen TL, Rasmussen BB, Flinois JP, Beaune P, Brosen K. In vitro metabolism of quinidine: the (3S)-3-hydroxylation of quinidine is a specific marker reaction for cytochrome P-4503A4 activity in human liver microsomes. J Pharmacol Exp Ther. 1999;289:31–37. [PubMed] [Google Scholar]
- 12.Marill J, Cresteil T, Lanotte M, Chabot GG. Identification of human cytochrome P450s involved in the formation of all-trans-retinoic acid principal metabolites. Mol Pharmacol. 2000;58:1341–1348. doi: 10.1124/mol.58.6.1341. [DOI] [PubMed] [Google Scholar]
- 13.Daly AK, Steen VM, Fairbrother KS, Idle JR. CYP2D6 multiallelism. Methods Enzymol. 1996;272:199–210. doi: 10.1016/s0076-6879(96)72024-4. [DOI] [PubMed] [Google Scholar]
- 14.Blin N, Stafford DW. A general method for isolation of high molecular weight DNA from eukaryotes. Nucl Acids Res. 1976;3:2303–2308. doi: 10.1093/nar/3.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mutch E, Blain PG, Williams FM. The role of metabolism in determining susceptibility to parathion toxicity in man. Toxicol Lett. 1999;107:177–187. doi: 10.1016/s0378-4274(99)00044-2. [DOI] [PubMed] [Google Scholar]
- 16.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T7. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lin YS, Dowling ALS, Quigley SD, et al. Co-regulation of CYP3A4 and CYP3A5 and contribution to hepatic and intestinal midazolam metabolism. Mol Pharmacol. 2002;62:162–172. doi: 10.1124/mol.62.1.162. [DOI] [PubMed] [Google Scholar]
- 18.Ozdemir V, Kalow W, Tang BK, et al. Evaluation of the genetic component of variability in CYP3A4 activity: a repeated drug administration method. Pharmacogenetics. 2000;10:373–388. doi: 10.1097/00008571-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Goodwin B, Redinbo MR, Kliewer SA. Regulation of CYP3A gene transcription by the pregnane X receptor. Ann Rev Pharmacol Toxicol. 2002;42:1–23. doi: 10.1146/annurev.pharmtox.42.111901.111051. [DOI] [PubMed] [Google Scholar]
- 20.Dai D, Tang J, Rose R, et al. Identification of variants of CYP3A4 and characterization of their abilities to metabolize testosterone and chlorpyrifos. J Pharmacol Exp Ther. 2001;299:825–831. [PubMed] [Google Scholar]
- 21.Lamba JK, Lin YS, Thummel K, et al. Common allelic variants of cytochrome P4503A4 and their prevalence in different populations. Pharmacogenetics. 2002;12:121–132. doi: 10.1097/00008571-200203000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Zhang J, Kuehl P, Green ED, et al. The human pregnane X receptor: genomic structure and identification and functional characterization of natural allelic variants. Pharmacogenetics. 2001;11:555–572. doi: 10.1097/00008571-200110000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Hustert E, Zibat A, Presecan-Siedel E, et al. Natural protein variants of pregnane X receptor with altered transactivation activity toward CYP3A4. Drug Metab Dispos. 2001;29:1454–1459. [PubMed] [Google Scholar]
- 24.Westlind A, Malmebo S, Johansson I, et al. Cloning and tissue distribution of a novel human cytochrome P450 of the CYP3A subfamily, CYP3A43. Biochem Biophys Res Commun. 2001;281:1349–1355. doi: 10.1006/bbrc.2001.4505. [DOI] [PubMed] [Google Scholar]