Abstract
Aims
The aim of this study was to characterize the population pharmacokinetics of levosimendan in patients with heart failure (NYHA grades III and IV) and its relationship to demographic factors, disease severity and concomitant use of digoxin and β-blocking agents.
Methods
Data from two efficacy studies with levosimendan administered by intravenous infusion were combined (190 patients in total). The data were analysed using a nonlinear mixed-effects modelling approach as implemented in the NONMEM program. The model development was done in three sequential steps. First the best structural model was determined (e.g. a one-, two- or three-compartment pharmacokinetic model). This was followed by the identification and incorporation of important covariates into the model. Lastly the stochastic part of the model was refined.
Results
A two-compartment model best described levosimendan pharmacokinetics. Clearance and the central volume of distribution were found to increase linearly with bodyweight. No other covariates, including concomitant use of digoxin and β-blocking agents, influenced the pharmacokinetics. In the final model, a 76-kg patient was estimated to have a clearance ± s.e. of 13.3 ± 0.4 l h−1 and a central volume of distribution of 16.8 ± 0.79 l. The interindividual variability was estimated to be 39% and 60% for clearance and central volume of distribution, respectively. Weight changed clearance by 1.5% [95% confidence interval (CI) 0.9%, 2.1%] and the central volume of distribution by 0.9% (95% CI 0.5%, 1.3%) per kg.
Conclusions
The population pharmacokinetics parameters of levosimendan in this patient group were comparable to those obtained by traditional methods in healthy volunteers and patients with mild heart failure. Bodyweight influenced the clearance and the central volume of distribution, which in practice is accounted for by weight adjusting doses. None of the other covariates, including digoxin and β-blocking agents, significantly influenced the pharmacokinetics of levosimendan.
Keywords: congestive heart failure, levosimendan, NONMEM, population pharmacokinetics
Introduction
Levosimendan Orion Corporation, Espoo, Finland is a novel calcium-sensitizing drug, which is intended for the treatment of heart failure. Its main mode of action is to increase the calcium sensitivity of troponin C, resulting in increased contractile force of the myocardium at lower calcium concentrations [1–3]. It has a favourable haemodynamic profile for use in heart failure, because it has positive inotropic and vasodilatory effects and is without negative effects on myocardial oxygen consumption and cardiac rhythm [4–7].
The pharmacokinetics of levosimendan have been studied in healthy volunteers and in patients with mild heart failure [8], and were best described by an open two-compartment model, predicting a short levosimendan half-life of only about 1 h. Levosimendan is highly bound to plasma proteins (97–98%) [8, 9]. Total clearance of levosimendan has been estimated at 200–360 ml min−1[10, 11]. Levosimendan undergoes extensive metabolism in man followed by excretion of metabolites in urine and faeces [12]. The main elimination pathway involves conjugation with glutathione. Through a minor elimination pathway two other metabolites are produced by reduction by anaerobic intestinal bacteria [10].
The aim of this study was to utilize plasma concentration data generated from two efficacy studies in patients with heart failure (NYHA grades III and IV), to determine the population pharmacokinetics of levosimendan and its relationship to demographic factors, disease severity and two important classes of concomitant medications—digoxin and β-blocking agents.
Methods
Data from two efficacy studies with levosimendan administered by intravenous infusion were combined. Multiple blood samples (about 10 per patient) were available from 190 patients. Sampling was spread over 0–60 h from the start of the treatment. The two studies were approved by the ethics committee of the Helsinki University Central Hospital and by the local ethics committee of each study site. All patients gave written informed consent before entering the study.
Chemical assay
Plasma concentrations of levosimendan were determined by high-performance liquid chromatography [13]. The relationship between concentration and detector response was linear and chromatograms contained no interfering peaks over the concentration range from 5 to 3000 ng ml−1. The within-run precision was < 10% at the quantification limit of 5 ng ml−1 and < 7% at higher concentrations. The between-run precision was < 7.4% at all concentrations tested.
Study protocols
The first study was a double-blind, placebo-controlled, multicentre dose-finding study of the effects of continuous infusion of levosimendan in patients with congestive cardiac failure [14]. In addition to the study drugs, all the patients received standard treatment with angiotensin-converting enzyme (ACE) inhibitors and diuretics. Patients were randomized to receive levosimendan, placebo, dobutamine (6 µg.kg min−1) or ethanol-containing vehicle, after right-sided catheterization. Levosimendan was given as a loading dose (10-min infusion, Table 1), followed by the same hourly dose given as a 24-h continuous infusion unless dose-limiting events occurred. Haemodynamics were monitored before drug administration and up to 26 h after the loading dose. Five different infusion rates of levosimendan (0.05 µg.kg min−1, 0.1 µg.kg min−1, 0.2 µg.kg min−1, 0.4 µg.kg min−1 and 0.6 µg.kg min−1) were compared with placebo and two open positive control groups receiving either ethanol or dobutamine. Blood samples (up to 11 per patient) were taken at various times after the start of the infusion. The intended sampling times were: 0, 0.5, 1, 2, 4, 8, 24, 24.5, 25 and 26 h after the start of the infusion. During the study the actual sampling times were recorded in the case record forms and these, rather than the intended sampling times, were used in the data analysis. The number of patients receiving levosimendan in this study was 95. Details are summarized in Table 1.
Table 1.
Details of the two studies included in the analysis.
| Study 1* | Study 2† | |
|---|---|---|
| Patient group | Stable CHF | Stable and unstable CHF |
| Study design | Randomized, double blind, parallel group | Randomized, double blind, parallel group |
| Study size | 151‡ | 146§ |
| Nominal sampling times¶ | 0, 0.5, 1, 2, 4, 8, 24, 24.5, 25, 26 | 0, 1, 2, 3, 4, 6, 24, 24.5, 25, 26, 28, 30, 48, 50/52**, 54 |
| Bolus dose (µg kg−1) | 3, 6, 12, 24, 36 | 6 |
| Infusion rates (µg.kg min−1) | 0.05, 0.1, 0.2, 0.4, 0.6 | 0.1, 0.2, 0.3, 0.4 |
| Infusion durations (h) | 24 | 6-48 |
The second study was a phase-III, multicentre, randomized, double-blind, placebo-controlled trial of intravenous levosimendan designed to determine its efficacy (acute and 48-h haemodynamic response) and safety in patients with congestive heart failure [15]. In addition to levosimendan, all patients received standard treatment with ACE inhibitors and diuretics. In the first period, after baseline haemodynamic measurements, patients were randomized to receive either intravenous levosimendan or placebo. Following a loading dose (6 µg kg−1 in 5 min) the patients received levosimendan in a maximum of four ascending doses (0.1, 0.2, 0.3 and 0.4 µg.kg min−1 for 55 min) at 1-h intervals until an adequate haemodynamic response occurred, or the patient had received the maximum dose, or had suffered a dose-limiting event. Thereafter, a maintenance infusion (at the appropriate dose) was continued for 6 h after the first dose. At this point, the randomization code was broken. In the second period, placebo patients were withdrawn from the study, whereas patients on levosimendan had their maximal doses halved. The infusion was continued for an additional 18 h (a total of 24 h). In a third period starting at 24 h after the first dose, patients were randomized to continue with levosimendan or to receive placebo for a further 24 h of monitoring of central haemodynamics. Blood samples for levosimendan concentrations were drawn at various times after the start and end of the infusion and after withdrawal. The intended sampling times were: 0, 1, 2, 3, 4, 6, 24, 24.5, 25, 26, 28, 30, 48, 50/52 (depending on randomization code) and 54 h after the start of the first infusion. During the study the actual sampling times were recorded in the case record forms and these, rather than the intended sampling times, were used in the data analysis. In total 98 actively treated patients were included in this study. Details are summarized in Table 1.
Information on sex, race (Asian, black, Caucasian, Hispanic and other), age, bodyweight, height, S-alanine aminotransferase (ALAT), S-aspartate aminotransferase (ASAT), S-alkaline phosphatase (ALKP), S-bilirubin (BILI), creatinine clearance (CRCL), as computed from the Cockroft and Gault formula [16], NYHA classification (III or IV), aetiology (coronary artery disease, non-ischaemic dilated cardiomyopathy and other) and concomitant use of β-blocking agents and digoxin (present or absent) was also collected. All covariates considered in the analyses are shown in Table 2 and Table 3.
Table 2.
Continuous covariates considered in the analysis.
| Covariate | Mean | Median | Range | n* |
|---|---|---|---|---|
| Age (years) | 60.6 | 61.0 | 30–84 | 193 |
| Height (cm) | 172.4 | 173 | 142–193 | 193 |
| Weight (kg) | 78.6 | 76.0 | 46.0–130.0 | 193 |
| ALAT (U l−1) | 23.3 | 19.5 | 6.0–97.0 | 193 |
| ASAT (U l−1) | 26.0 | 23.0 | 11.0–65.0 | 95 |
| ALKP (U l−1) | 131 | 112 | 19.0–376.0 | 176 |
| CREA (µ) | 111 | 106 | 53.0–336.0 | 189 |
| BILI (mg dl−1) | 1.13 | 0.950 | 0.1–4.0 | 95 |
| CRCL (ml min−1) | 72.0 | 71.0 | 46.0–130.0 | 189 |
Number of patients with reported values. Missing values were imputed with the median covariate value. ALAT, S-alanine aminotransferase; ASAT, S-aspartate aminotransferase; ALKP, S-alkaline phosphatase; BILI, S-bilirubin.
Table 3.
Categorical covariates (n = 193) considered in the analysis.
| Covariate | Count |
|---|---|
| Sex | |
| Male | 161 |
| Female | 32 |
| Race | |
| Asian | 2 |
| Black | 33 |
| Caucasian | 154 |
| Hispanic | 3 |
| Other | 1 |
| New York Heart Association score | |
| Class 3 | 157 |
| Class 4 | 36 |
| Digoxin | |
| With | 106 |
| Without | 87 |
| β-blocking agent | |
| With | 32 |
| Without | 161 |
| Aetiology | |
| Coronary artery disease | 156 |
| Non-ischaemic dilated cardiomyopathy | 32 |
| Other | 5 |
Pharmacokinetic modelling
The data were analysed using a nonlinear mixed effects modelling approach. In a nonlinear mixed effects model it is possible to quantify both unexplained inter- and intra-subject variability (random effects) as well as the influence of measured concomitant effects or covariates (fixed effects) on basic model parameters [17]. It is also possible to utilize sparse data that would not allow individual modelling using traditional methods. The most commonly used program to analyse nonlinear mixed effects models is NONMEM [18]. In the current analysis we used NONMEM version V together with the S-PLUS (Insightful Corp., Seattle, WA, USA)-based program Xpose version 2.0 [19] for goodness of fit assessment and covariate model building. The first-order estimation method in NONMEM was used for all analyses.
Model development strategy
The following strategy was used to develop the final model. First, the structural pharmacokinetic model was developed (e.g. a one-, two- or a three-compartment model). Important covariates were then identified and incorporated into the model. Last, the statistical model (random effects) was refined.
Structural pharmacokinetic model development
The appropriate structural model was developed by examination of goodness of fit graphs together with changes in the objective function value (OFV) computed by NONMEM. The OFV is proportional to the −2 times the log-likelihood of the data, given the model, and therefore, the difference in OFV (ΔOFV) between two hierarchical models is approximately χ2-distributed. A ΔOFV of 10.8 between the competing models (d.f. = 1), corresponding to a nominal P-value of < 0.001, was required for the more complex model to be regarded as significantly better than the less complex one. This rather strict criterion was motivated by the multiple testing involved in the model development as well as the fact that the ΔOFV is only approximately χ2-distributed [20].
Covariate model development
Exploratory graphical analysis and a GAM analysis were used for the identification of important covariates explaining interindividual variability in the pharmacokinetic parameters [21]. The term GAM refers to a stepwise, generalized additive modelling procedure implemented with Xpose 2.0. This utilizes the individual (empirical Bayes) parameter estimates from a NONMEM analysis without any covariate effects in the model (the basic model). The GAM finds the subset of available explanatory parameters most useful in explaining variability of the parameter, by testing combinations of models of the covariates in a stepwise fashion. The covariates are usually assumed to enter the function in an additive fashion and can be either linearly or nonlinearly related to the parameters. In the GAM analysis categorical covariates with more than two levels were reduced to two levels by pooling the groups with the smallest number of individuals into those with the largest number of individuals. For example, Asian (n = 2), Hispanic (n = 3) and others (n = 1) were pooled with Caucasians. The reason for this pooling was to avoid obtaining spurious candidate covariate relationships. If the GAM identified any of these categorical covariates as important, the original levels were used when the covariate was tested in NONMEM. The Aikaike information criterion (AIC) was used to discriminate between models.
The candidate covariates identified in the GAM analysis were then tested in NONMEM. They were included into the basic population model to form the full model. The relative importance of the individual covariate terms was assessed by deleting them one at a time from the full model and noting the change in objective function. The least important covariate, if it was not statistically significant (a ΔOFV of 10.8 corresponding to a nominal P-value of < 0.001), was discarded and the importance of the remaining covariates was re-assessed in the revised model. This backward deletion continued until all the remaining covariate effects were significant. To remain in the final model a covariate had to be significant at the nominal significance level (P < 0.001) and the functional form (e.g. the sign of the slope) should not have changed from what was found in the GAM.
Statistical model development
Exponential distribution models were used to account for interindividual variability. A full Ω matrix (i.e. estimating correlations between all parameters) was used during the covariate model building. After this, the model was modified to include only the correlations that gave a ΔOFV of 10.8.
The residual error model was determined by examination of goodness of fit plots. Those considered were the proportional error model, the slope intercept error model and the additive error model on log-transformed data.
Results
Levosimendan concentrations were obtained for 190 of the 193 patients who received the drug. The total number of observations was 1793 (768 and 1025 in the first and second study, respectively). Two patients had drug concentrations that deviated substantially and erratically from what would be expected from the model and dosing scheme. Thus, it was not possible to handle any single observation from these two patients in the same way as other outliers (see below). These two individuals were omitted completely from the analysis. During the model development, it was decided to omit 41 of the reported drug concentrations. The reasons for omission were either drug detected in plasma prior to drug administration (5 points), increasing drug concentrations after the termination of the infusion (18 points), and unexpectedly high concentrations relative to other values in the patient (18 points). The parameter estimates obtained when the final model was re-estimated with these observations returned to the data set differed only marginally from the estimates based on the reduced data set, although the residual error increased from 25% to 33%.
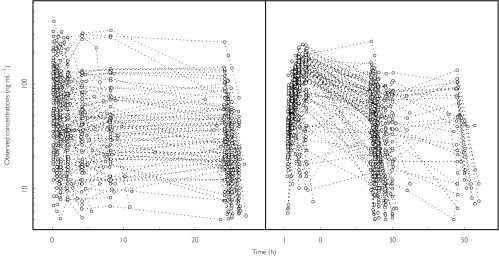
Levosimendan concentrations vs time after dose are shown in Figure 1 and a summary of the observed covariates in Table 2 (continuous) and Table 3 (categorical).
Figure 1.
Individual plasma levosimendan concentration vs time data. Values from each subject are connected with a line. Left panel, study 1; right panel, study 2.
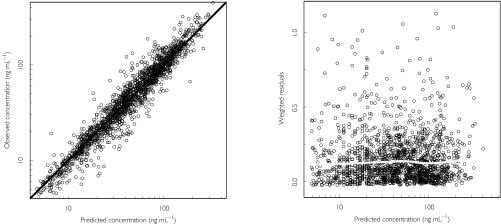
A two-compartment pharmacokinetic model with zero-order input and first-order elimination was found to describe the data better than a one-compartment model and no worse than a three-compartment model. There was no indication of saturable elimination or other nonlinearities in the goodness of fit graphs, which is in line with conclusions based on healthy volunteer data [22]. Figure 2 shows basic goodness of fit plots for the final levosimendan model. The left panel provides information on the appropriateness of the structural model. Evidence of a good fit is the even spread of the data around the line of identity (the solid diagonal line). The right panel provides diagnostics for the residual error model. The two-compartment pharmacokinetic model was parameterized in terms of clearance (CL), intercompartmental clearance (Q) and the volumes of distributions of the central and peripheral compartments (V1 and V2, respectively).
Figure 2.
Goodness-of-fit plots for the final model. The left panel shows the observed vs the predicted concentrations based on the individual parameter estimates. The thick diagonal line is the line of identity. The right panel is a plot of the absolute values of the individually weighted residuals vs the individual predictions. The solid, white and approximately horizontal line shows the results of the nonparametric smooth regression analysis.
The GAM analysis identified sex, concomitant digoxin use, bodyweight and BILI as potential covariates on CL; sex, bodyweight, concomitant use of β-blocking agents, BILI and ALKP as potential covariates on V1; and sex, age and concomitant use of digoxin as potential covariates on Q. These were added to the basic model and deleted one at a time as described in Methods (Table 4). The final covariate model retained only bodyweight on CL and V1.
Table 4.
Results from the deletion of covariate relations from the full model
| Step | Model | ΔOFV |
|---|---|---|
| Full model | – | |
| 1 | Minus age on Q | 0.3 |
| 2 | Minus digoxin on Q | 0.3 |
| 3 | Minus β-blocking agents on V1 | 0.7 |
| 4 | Minus sex on V1 | 1.2 |
| 5 | Minus digoxin on CL | 5.4 |
| 6 | Minus sex on Q | 8.5 |
| 7 | Minus sex on CL | 7.5 |
| 8 | Minus bodyweight on CL* | 35.9 |
| 9 | Minus body weight on V1* | 11.9 |
Covariates relation retained in the model.
The residual error was modelled using a log-transformation as indicated by the skewed distribution of the weighted residuals from the basic model without any covariates. The right panel in Figure 2 shows that this transformation adequately handles the residual error distribution. The data are evenly spread over the individual predictions and the smooth nonparametric regression line is approximately horizontal.
Proportional models for interindividual variability were used for all pharmacokinetic parameters. A full covariance matrix, estimating covariance between all parameters, was used during the covariate model building. However, after refinement of the covariate model, further statistical model building indicated that one including only covariance terms for CL, Q and V1 was the most suitable.
The final model was a two-compartment open model with weight influencing CL and V1. The interindividual variability model included interindividual variability terms for all basic pharmacokinetic parameters (CL, V1, Q and V2) as well as terms for the covariance structure between CL, V1 and Q. The parameter values ± s.e. for a person with weight of 76 kg were 13.3 ± 0.4 l h−1 for CL, 16.8 ± 0.8 l for V1, 2.33 ± 0.3 l h−1 for Q and 30.0 ± 6 l for V2. Weight increased (decreased) CL by 1.5% [95% confidence interval (CI) 0.9%, 2.1%] and V1 by 0.9% (95% CI 0.5%, 1.3%) per kg. The parameter estimates for the final model are summarized in Table 5. The mean ± s.d. terminal half-life computed from the empirical Bayes estimates of the pharmacokinetic parameters was 0.84 ± 0.3 h.
Table 5.
Parameter estimates from the final model.
| Parameter | Estimate (% R.SE*) |
|---|---|
| Structural model parameters | |
| CL (L h−1) | 13.3† (3.2) |
| Bodyweight on CL (% kg−1) | 1.5 (20) |
| V1 (l) | 16.8† (4.7) |
| Bodyweight on V1 (% kg−1) | 0.9 (26) |
| Q (l h−1) | 2.3 (12) |
| V2 (l) | 30.0 (20) |
| Variability parameters | |
| CL (%) | 39 (14‡) |
| Correlation CL-V1 | 0.47 (23§) |
| Correlation CL-Q | 0.25 (47§) |
| V1 (%) | 60 (18‡) |
| Correlation V1-Q | −0.4 (40§) |
| Q (%) | 170 (30‡) |
| V2 (%) | 260 (43‡) |
| Residual error (%) | 25 (7.8‡) |
Relative standard error.
Patient weighing 76 kg.
The R.SE (%) for the corresponding variance term.
The R.SE (%) for the corresponding covariance term.
Discussion
The present analysis shows that the pharmacokinetics of levosimendan in moderate and severe heart failure patients are the same as those from healthy volunteers and patients with mild heart failure [8, 9]. In healthy volunteers and patients with mild heart failure CL was estimated to be between 200 and 360 ml min−1 (corresponding to a range of 12–22 l h−1), which is in good agreement with the mean value of 13.3 l h−1 obtained in the present analysis. Similarly, the half-life in the former group was about 1 h, and in the present study 0.8 h. The pharmacokinetic parameters were also similar after continuous infusion up to 48 h compared with those obtained after bolus doses of levosimendan [8, 11], indicating that the metabolism of levosimendan is not saturated over the dose range.
The influence of various covariates on the pharmacokinetics of levosimendan was assessed. Congestive heart failure is known to alter the pharmacokinetics of many drugs [23]. The reduction in central volume and fluid retention within cells might lead to alterations in drug distribution and increased drug concentrations. However, the severity of heart failure did not influence the pharmacokinetics of levosimendan in this study of patients with NYHA class III and IV disease. The pharmacokinetics was also similar regardless of the aetiology of disease (coronary artery disease or non-ischaemic dilated cardiomyopathy).
The current medical therapy for heart failure patients includes angiotensin converting enzyme inhibitors, diuretics, cardiac glycosides and β-blockers. In the present analysis, concomitant medication with digoxin and β-blockers was included as covariate. Neither of these drugs was found to affect levosimendan pharmacokinetics.
Renal function is often impaired in heart failure patients. This can lead to diminished excretion of drugs, possibly requiring a dosage change. Although creatinine clearance was not directly determined from the patients taking part in the two studies, the formula of Cockroft and Gault [24] was used to calculate values from the baseline serum creatinine. The present analysis did not reveal any difference in pharmacokinetics of levosimendan in patients in which the degree of renal function varied between moderate impairment to normal (46–130 ml min−1). This is in accordance with previous studies in which the pharmacokinetics of unchanged levosimendan was similar in healthy subjects and in patients with mild to moderate renal failure [25].
CL and V1 were found to increase with increasing bodyweight, which confirms the rationale of dosing levosimendan according to bodyweight. No other covariates (age, race gender or hepatic function) influenced the pharmacokinetics of levosimendan.
In conclusion, the pharmacokinetic parameters of levosimendan assessed by the population approach are the same as those obtained by traditional methods. The parameters after continuous infusion were also similar to those obtained after a bolus dose. No significant changes in pharmacokinetics of levosimendan were seen in different population subgroups. The only covariate that was found to affect the pharmacokinetics of levosimendan was weight.
Acknowledgments
The study was sponsored by Orion Corporation, Espoo, Finland.
References
- 1.Haikala H, Kaivola J, Nissinen E, Wall P, Levijoki J, Linden IB. Cardiac troponin C as a target protein for a novel calcium sensitizing drug, levosimendan. J Mol Cell Cardiol. 1995;27:1859–1866. doi: 10.1016/0022-2828(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 2.Haikala H, Levijoki J, Linden IB. Troponin C-mediated calcium sensitization by levosimendan accelerates the proportional development of isometric tension. J Mol Cell Cardiol. 1995;27:2155–2165. doi: 10.1016/s0022-2828(95)91371-8. [DOI] [PubMed] [Google Scholar]
- 3.Pollesello P, Ovaska M, Kaivola J, et al. Binding of a new Ca2+ sensitizer, levosimendan, to recombinant human cardiac troponin C. A molecular modelling, fluorescence probe, and proton nuclear magnetic resonance study. J Biol Chem. 1994;269:28584–28590. [PubMed] [Google Scholar]
- 4.Yokoshiki H, Katsube Y, Sunagawa M, Sperelakis N. The novel calcium sensitizer levosimendan activates the ATP-sensitive K+ channel in rat ventricular cells. J Pharmacol Exp Ther. 1997;283:375–383. [PubMed] [Google Scholar]
- 5.Lilleberg J, Nieminen MS, Akkila J, et al. Effects of a new calcium sensitizer, levosimendan, on haemodynamics, coronary blood flow and myocardial substrate utilization early after coronary artery bypass grafting. Eur Heart J. 1998;19:660–668. doi: 10.1053/euhj.1997.0806. [DOI] [PubMed] [Google Scholar]
- 6.Ukkonen H, Saraste M, Akkila J, et al. Myocardial efficiency during levosimendan infusion in congestive heart failure. Clin Pharmacol Ther. 2000;68:522–531. doi: 10.1067/mcp.2000.110972. [DOI] [PubMed] [Google Scholar]
- 7.Singh BN, Lilleberg J, Sandell E-P, Ylönen V, Lehtonen L, Toivonen L. Effects of levosimendan on cardiac arrhythmia. electrophysiologic and ambulatory electrocardiographic findings in phase II and phase III clinical studies in cardiac failure. Am J Cardiol. 1999;83:16(I)–20(I). [Google Scholar]
- 8.Sandell EP, Hayha M, Antila S, et al. Pharmacokinetics of levosimendan in healthy volunteers and patients with congestive heart failure. J Cardiovasc Pharmacol. 1995;26:S57–S62. [PubMed] [Google Scholar]
- 9.Antila S, Jarvinen A, Honkanen T, Lehtonen L. Pharmacokinetic and pharmacodynamic interactions between the novel calcium sensitiser levosimendan and warfarin. Eur J Clin Pharmacol. 2000;56:705–710. doi: 10.1007/s002280000204. [DOI] [PubMed] [Google Scholar]
- 10.Antila S, Jarvinen A, Akkila J, Honkanen T, Karlsson M, Lehtonen L. Studies on psychomotoric effects and pharmacokinetic interactions of the new calcium sensitizing drug levosimendan and ethanol. Arzneimittelforschung. 1997;47:816–820. [PubMed] [Google Scholar]
- 11.Sundberg S, Antila S, Scheinin H, Hayha M, Virtanen M, Lehtonen L. Integrated pharmacokinetics and pharmacodynamics of the novel calcium sensitizer levosimendan as assessed by systolic time intervals. Int J Clin Pharmacol Ther. 1998;36:629–635. [PubMed] [Google Scholar]
- 12.Lehtonen LA. Levosimendan: a parenteral calcium-sensitising drug with additional vasodilatory properties. Expert Opin Invest Drugs. 2001;10:955–970. doi: 10.1517/13543784.10.5.955. [DOI] [PubMed] [Google Scholar]
- 13.Karlsson M, Korkolainen T, Wikberg T. Automated analysis of levosimendan in human plasma by on-line dialysis and liquid chromatography. Biomed Chromatogr. 1997;11:54–58. doi: 10.1002/(SICI)1099-0801(199701)11:1<54::AID-BMC629>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 14.Nieminen MS, Akkila J, Hasenfuss G, et al. Hemodynamic and neurohumoral effects of continuous infusion of levosimendan in patients with congestive heart failure. J Am Coll Cardiol. 2000;36:1903–1912. doi: 10.1016/s0735-1097(00)00961-x. [DOI] [PubMed] [Google Scholar]
- 15.Slawsky MT, Colucci WS, Gottlieb SS, et al. Acute hemodynamic and clinical effects of levosimendan in patients with severe heart failure. Study Invest Circulation. 2000;102:2222–2227. doi: 10.1161/01.cir.102.18.2222. [DOI] [PubMed] [Google Scholar]
- 16.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 17.Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8:195–222. [PubMed] [Google Scholar]
- 18.Beal SL, Sheiner LB. NONMEM Users Guide. San Fransisco: University of California; 1992. [Google Scholar]
- 19.Jonsson EN, Karlsson MO. Xpose—an S-PLUS based population pharmacokinetic/pharmacodynamic model building aid for NONMEM. Comput Meth Programs Biomed. 1999;58:51–64. doi: 10.1016/s0169-2607(98)00067-4. [DOI] [PubMed] [Google Scholar]
- 20.Wählby U, Jonsson EN, Karlsson MO. Assessment of actual significance levels for covariate effects in NONMEM. J Pharmacokinet Pharmacodyn. 2001;28:231–252. doi: 10.1023/a:1011527125570. [DOI] [PubMed] [Google Scholar]
- 21.Mandema JW, Verotta D, Sheiner LB. Building population pharmacokinetic-pharmacodynamic models. I. Models for covariate effects. J Pharmacokinet Biopharm. 1992;20:511–528. doi: 10.1007/BF01061469. [DOI] [PubMed] [Google Scholar]
- 22.Lilleberg J, Antila S, Karlsson M, Nieminen MS, Pentkainen PJ. Pharmacokinetics and pharmacodynamics of simendan, a novel calcium sensitizer, in healthy volunteers. Clin Pharmacol Ther. 1994;56:554–563. doi: 10.1038/clpt.1994.177. [DOI] [PubMed] [Google Scholar]
- 23.Shammas FV, Dickstein K. Clinical pharmacokinetics in heart failure. An updated review. Clin Pharmacokinet. 1988;15:94–113. doi: 10.2165/00003088-198815020-00002. [DOI] [PubMed] [Google Scholar]
- 24.Lafayette RA, Perrone RD, Levey AS. Laboratory evaluation of renal function. In Diseases of the Kidney. In: Schrier RW, Gottschalk CW, editors. 6. Boston, New York, Toronto, London: Little, Brown and Co; 1997. pp. 314–319. [Google Scholar]
- 25.Sandell E, Antila S, Koistinen H, Pentikäinen P. The effects of renal failure on the pharmacokinetics of levosimendan. Thérapie. 1995;(Suppl):A495. [Google Scholar]